Genetic reduction of striatal-enriched tyrosine phosphatase (STEP) reverses cognitive and cellular deficits in an Alzheimer's disease mouse model
- PMID: 20956308
- PMCID: PMC2973892
- DOI: 10.1073/pnas.1013543107
Genetic reduction of striatal-enriched tyrosine phosphatase (STEP) reverses cognitive and cellular deficits in an Alzheimer's disease mouse model
Abstract
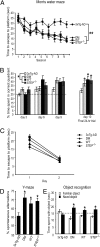
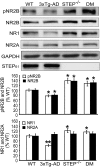
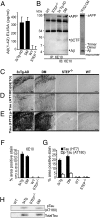
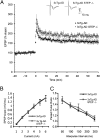
Alzheimer's disease (AD) is a progressive and incurable neurodegenerative disorder. Early in the pathophysiology of AD, synaptic function is disrupted by soluble Aβ oligomers, possibly through Aβ-mediated internalization of NMDA receptors. Striatal-enriched phosphatase (STEP) is a tyrosine phosphatase that regulates the internalization of NMDA receptors. Recent work shows that STEP is elevated in the prefrontal cortex of human AD patients and in animal models of AD. Here, we use genetic manipulations to reduce STEP activity in a triple transgenic AD mouse model and show that a decrease in STEP levels reverses cognitive and cellular deficits observed in these mice. Our results suggest that STEP inhibitors may prove therapeutic for this devastating disorder.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Inhibitor of the tyrosine phosphatase STEP reverses cognitive deficits in a mouse model of Alzheimer's disease.PLoS Biol. 2014 Aug 5;12(8):e1001923. doi: 10.1371/journal.pbio.1001923. eCollection 2014 Aug. PLoS Biol. 2014. PMID: 25093460 Free PMC article.
-
Abeta-mediated NMDA receptor endocytosis in Alzheimer's disease involves ubiquitination of the tyrosine phosphatase STEP61.J Neurosci. 2010 Apr 28;30(17):5948-57. doi: 10.1523/JNEUROSCI.0157-10.2010. J Neurosci. 2010. PMID: 20427654 Free PMC article.
-
Reduced levels of the tyrosine phosphatase STEP block β amyloid-mediated GluA1/GluA2 receptor internalization.J Neurochem. 2011 Nov;119(3):664-72. doi: 10.1111/j.1471-4159.2011.07450.x. Epub 2011 Sep 21. J Neurochem. 2011. PMID: 21883219 Free PMC article.
-
Striatal-enriched Tyrosine Protein Phosphatase (STEP) in the Mechanisms of Depressive Disorders.Curr Protein Pept Sci. 2017 Aug 30;18(11):1152-1162. doi: 10.2174/1389203718666170710121532. Curr Protein Pept Sci. 2017. PMID: 28699511 Review.
-
APP transgenic modeling of Alzheimer's disease: mechanisms of neurodegeneration and aberrant neurogenesis.Brain Struct Funct. 2010 Mar;214(2-3):111-26. doi: 10.1007/s00429-009-0232-6. Epub 2009 Nov 29. Brain Struct Funct. 2010. PMID: 20091183 Free PMC article. Review.
Cited by
-
Fragile X mental retardation protein: from autism to neurodegenerative disease.Front Cell Neurosci. 2015 Feb 12;9:43. doi: 10.3389/fncel.2015.00043. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25729352 Free PMC article. No abstract available.
-
Translational Assays for Assessment of Cognition in Rodent Models of Alzheimer's Disease and Dementia.J Mol Neurosci. 2016 Nov;60(3):371-382. doi: 10.1007/s12031-016-0837-1. Epub 2016 Sep 16. J Mol Neurosci. 2016. PMID: 27637601 Review.
-
Striatal-enriched protein tyrosine phosphatase modulates nociception: evidence from genetic deletion and pharmacological inhibition.Pain. 2016 Feb;157(2):377-386. doi: 10.1097/j.pain.0000000000000329. Pain. 2016. PMID: 26270590 Free PMC article.
-
Pharmacological inhibition of STriatal-Enriched protein tyrosine Phosphatase by TC-2153 reduces hippocampal excitability and seizure propensity.Epilepsia. 2022 May;63(5):1211-1224. doi: 10.1111/epi.17192. Epub 2022 Feb 21. Epilepsia. 2022. PMID: 35188269 Free PMC article.
-
The Non-receptor Tyrosine Kinase Pyk2 in Brain Function and Neurological and Psychiatric Diseases.Front Synaptic Neurosci. 2021 Oct 6;13:749001. doi: 10.3389/fnsyn.2021.749001. eCollection 2021. Front Synaptic Neurosci. 2021. PMID: 34690733 Free PMC article. Review.
References
-
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. - PubMed
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 NS033020/NS/NINDS NIH HHS/United States
- R37 NS033020-19/NS/NINDS NIH HHS/United States
- P01 AG009464/AG/NIA NIH HHS/United States
- R01 NS042304/NS/NINDS NIH HHS/United States
- NS42304/NS/NINDS NIH HHS/United States
- MH52711/MH/NIMH NIH HHS/United States
- K02 MH001527/MH/NIMH NIH HHS/United States
- K08 MH081190/MH/NIMH NIH HHS/United States
- R01 AG034924/AG/NIA NIH HHS/United States
- NS39962/NS/NINDS NIH HHS/United States
- AG09464/AG/NIA NIH HHS/United States
- R01 NS039962/NS/NINDS NIH HHS/United States
- R01 MH052711/MH/NIMH NIH HHS/United States
- MH081190/MH/NIMH NIH HHS/United States
- MH01527/MH/NIMH NIH HHS/United States
- R01 AG034924-01A1/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

