Association of nuclear localization of a long interspersed nuclear element-1 protein in breast tumors with poor prognostic outcomes
- PMID: 20948976
- PMCID: PMC2952938
- DOI: 10.1177/1947601909360812
Association of nuclear localization of a long interspersed nuclear element-1 protein in breast tumors with poor prognostic outcomes
Abstract
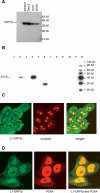
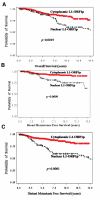
Within healthy human somatic cells, retrotransposition by long interspersed nuclear element-1 (also known as LINE-1 or L1) is thought to be held in check by a variety of mechanisms, including DNA methylation and RNAi. The expression of L1-ORF1 protein, which is rarely found in normal tissue, was assayed using antibodies with a variety of clinical cancer specimens and cancer cell lines. L1-ORF1p expression was detected in nearly all breast tumors that the authors examined, and the protein was also present in a high percentage of ileal carcinoids, bladder, and pancreatic neuroendocrine tumors, as well as in a smaller percentage of prostate and colorectal tumors. Tumors generally demonstrated cytoplasmic L1-ORF1p; however, in several breast cancers, L1-ORF1p was nuclear. Patients with breast tumors displaying nuclear L1-ORF1p had a greater incidence of both local recurrence and distal metastases and also showed poorer overall survival when compared with patients with tumors displaying cytoplasmic L1-ORF1p. These data suggest that expression of L1-ORF1p is widespread in many cancers and that redistribution from cytoplasm to nucleus could be a poor prognostic indicator during breast cancer. High expression and nuclear localization of L1-ORF1p may result in a higher rate of L1 retrotransposition, which could increase genomic instability.
Keywords: breast cancers; line expression; line movement.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures


Similar articles
-
Prognostic value of LINE-1 retrotransposon expression and its subcellular localization in breast cancer.Breast Cancer Res Treat. 2012 Nov;136(1):129-42. doi: 10.1007/s10549-012-2246-7. Epub 2012 Sep 29. Breast Cancer Res Treat. 2012. PMID: 23053642 Free PMC article.
-
Identification of charged amino acids required for nuclear localization of human L1 ORF1 protein.Mob DNA. 2019 May 6;10:20. doi: 10.1186/s13100-019-0159-2. eCollection 2019. Mob DNA. 2019. PMID: 31080522 Free PMC article.
-
Characterization of L1 ORF1p self-interaction and cellular localization using a mammalian two-hybrid system.PLoS One. 2013 Dec 4;8(12):e82021. doi: 10.1371/journal.pone.0082021. eCollection 2013. PLoS One. 2013. PMID: 24324740 Free PMC article.
-
Protein-nucleic acid interactions of LINE-1 ORF1p.Semin Cell Dev Biol. 2019 Feb;86:140-149. doi: 10.1016/j.semcdb.2018.03.019. Epub 2018 Mar 31. Semin Cell Dev Biol. 2019. PMID: 29596909 Free PMC article. Review.
-
Guardian of the Human Genome: Host Defense Mechanisms against LINE-1 Retrotransposition.Front Chem. 2016 Jun 28;4:28. doi: 10.3389/fchem.2016.00028. eCollection 2016. Front Chem. 2016. PMID: 27446907 Free PMC article. Review.
Cited by
-
Long interspersed element-1 (LINE-1): passenger or driver in human neoplasms?PLoS Genet. 2013 Mar;9(3):e1003402. doi: 10.1371/journal.pgen.1003402. Epub 2013 Mar 28. PLoS Genet. 2013. PMID: 23555307 Free PMC article. Review.
-
Inhibition of LINE-1 retrotransposon-encoded reverse transcriptase modulates the expression of cell differentiation genes in breast cancer cells.Breast Cancer Res Treat. 2014 Jan;143(2):239-53. doi: 10.1007/s10549-013-2812-7. Epub 2013 Dec 12. Breast Cancer Res Treat. 2014. PMID: 24337508 Free PMC article.
-
Patterns of Transposable Element Expression and Insertion in Cancer.Front Mol Biosci. 2016 Nov 16;3:76. doi: 10.3389/fmolb.2016.00076. eCollection 2016. Front Mol Biosci. 2016. PMID: 27900322 Free PMC article.
-
A conserved role for the ESCRT membrane budding complex in LINE retrotransposition.PLoS Genet. 2017 Jun 6;13(6):e1006837. doi: 10.1371/journal.pgen.1006837. eCollection 2017 Jun. PLoS Genet. 2017. PMID: 28586350 Free PMC article.
-
A droplet digital PCR detection method for rare L1 insertions in tumors.Mob DNA. 2014 Dec 31;5(1):30. doi: 10.1186/s13100-014-0030-4. eCollection 2014. Mob DNA. 2014. PMID: 25598847 Free PMC article.
References
-
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, et al. Initial sequencing and analysis of the human genome. Nature 2001;409:860-921 - PubMed
-
- Babushok DV, Kazazian HH., Jr Progress in understanding the biology of the human mutagen LINE-1. Hum Mutat 2007;28:527-39 - PubMed
-
- Kulpa DA, Moran JV. Ribonucleoprotein particle formation is necessary but not sufficient for LINE-1 retrotransposition. Hum Mol Genet 2005;14:3237-48 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
