Dependency of γ-secretase complex activity on the structural integrity of the bilayer
- PMID: 20937251
- PMCID: PMC2981652
- DOI: 10.1016/j.bbrc.2010.10.017
Dependency of γ-secretase complex activity on the structural integrity of the bilayer
Abstract
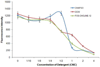
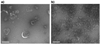
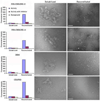
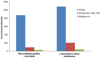
γ-secretase is a membrane protein complex associated with the production of Aβ peptides that are pathogenic in Alzheimer's disease. We have characterized the activity of γ-secretase complexes under a variety of detergent solubilization and reconstitution conditions, and the structural state of proteoliposomes by electron microscopy. We found that γ-secretase activity is highly dependent on the physical state or integrity of the membrane bilayer--partial solubilization may increase activity while complete solubilization will abolish it. The activity of well-solubilized γ-secretase can be restored to near native levels when properly reconstituted into a lipid bilayer environment.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Rat brain gamma-secretase activity is highly influenced by detergents.Biochemistry. 2007 Jun 26;46(25):7647-54. doi: 10.1021/bi0621258. Epub 2007 May 31. Biochemistry. 2007. PMID: 17536783
-
Presenilin 1 is linked with gamma-secretase activity in the detergent solubilized state.Proc Natl Acad Sci U S A. 2000 May 23;97(11):6138-43. doi: 10.1073/pnas.110126897. Proc Natl Acad Sci U S A. 2000. PMID: 10801983 Free PMC article.
-
Phosphoinositides suppress gamma-secretase in both the detergent-soluble and -insoluble states.J Biol Chem. 2008 Jul 11;283(28):19283-92. doi: 10.1074/jbc.M705954200. Epub 2008 May 14. J Biol Chem. 2008. PMID: 18480063
-
Isolation of intramembrane proteases in membrane-like environments.Biochim Biophys Acta Biomembr. 2020 Apr 1;1862(4):183193. doi: 10.1016/j.bbamem.2020.183193. Epub 2020 Jan 13. Biochim Biophys Acta Biomembr. 2020. PMID: 31945321 Review.
-
Membrane trafficking and proteolytic activity of γ-secretase in Alzheimer's disease.Biol Chem. 2016 Sep 1;397(9):827-35. doi: 10.1515/hsz-2016-0146. Biol Chem. 2016. PMID: 27390881 Review.
Cited by
-
Active site geometry stabilization of a presenilin homolog by the lipid bilayer promotes intramembrane proteolysis.Elife. 2022 May 17;11:e76090. doi: 10.7554/eLife.76090. Elife. 2022. PMID: 35579427 Free PMC article.
-
Generation of Alzheimer disease-associated amyloid β42/43 peptide by γ-secretase can be inhibited directly by modulation of membrane thickness.J Biol Chem. 2012 Jun 15;287(25):21326-34. doi: 10.1074/jbc.M112.356659. Epub 2012 Apr 24. J Biol Chem. 2012. PMID: 22532566 Free PMC article.
-
High-efficient production and biophysical characterisation of nicastrin and its interaction with APPC100.Sci Rep. 2017 Mar 9;7:44297. doi: 10.1038/srep44297. Sci Rep. 2017. PMID: 28276527 Free PMC article.
References
-
- Li YM, Xu M, Lai MT, Huang Q, Castro JL, DiMuzio-Mower J, Harrison T, Lellis C, Nadin A, Neduvelil JG, Register RB, Sardana MK, Shearman MS, Smith AL, Shi XP, Yin KC, Shafer JA, Gardell SJ. Photoactivated γ-secretase inhibitors directed to the active site covalently label presenilin 1. Nature. 2000;405:689–694. - PubMed
-
- Esler WP, Wolfe MS. A portrait of Alzheimer secretases-new features and familiar faces. Science. 2001;293:1449–1454. - PubMed
-
- Periz G, Fortini ME. Functional reconstitution of gamma-secretase through coordinated expression of presenilin, nicastrin, Aph-1, and Pen-2. J. Neurosci. Res. 2004;77:309–322. - PubMed
-
- Wolfe MS. The gamma-secretase complex: membrane-embedded proteolytic ensemble. Biochemistry. 2006;45:7931–7939. - PubMed
-
- Thinakaran G, Borchelt DR, Lee MK, Slunt HH, Spitzer L, Kim G, Ratovitsky T, Davenport F, Nordstedt C, Seeger M, Hardy J, Levey AI, Gandy SE, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron. 1996;17:181–190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

