Genetic background modifies neurodegeneration and neuroinflammation driven by misfolded human tau protein in rat model of tauopathy: implication for immunomodulatory approach to Alzheimer's disease
- PMID: 20937161
- PMCID: PMC2958906
- DOI: 10.1186/1742-2094-7-64
Genetic background modifies neurodegeneration and neuroinflammation driven by misfolded human tau protein in rat model of tauopathy: implication for immunomodulatory approach to Alzheimer's disease
Abstract
Background: Numerous epidemiological studies demonstrate that genetic background modifies the onset and the progression of Alzheimer's disease and related neurodegenerative disorders. The efficacious influence of genetic background on the disease pathway of amyloid beta has been meticulously described in rodent models. Since the impact of genetic modifiers on the neurodegenerative and neuroinflammatory cascade induced by misfolded tau protein is yet to be elucidated, we have addressed the issue by using transgenic lines expressing the same human truncated tau protein in either spontaneously hypertensive rat (SHR) or Wistar-Kyoto (WKY) genetic background.
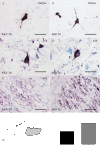
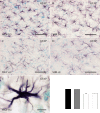
Methods: Brains of WKY and SHR transgenic rats in the terminal stage of phenotype and their age-matched non-transgenic littermates were examined by means of immunohistochemistry and unbiased stereology. Basic measures of tau-induced neurodegeneration (load of neurofibrillary tangles) and neuroinflammation (number of Iba1-positive microglia, their activated morphology, and numbers of microglia immunoreactive for MHCII and astrocytes immunoreactive for GFAP) were quantified with an optical fractionator in brain areas affected by neurofibrillary pathology (pons, medulla oblongata). The stereological data were evaluated using two-way ANOVA and Student's t-test.
Results: Tau neurodegeneration (neurofibrillary tangles (NFTs), axonopathy) and neuroinflammation (microgliosis, astrocytosis) appeared in both WKY and SHR transgenic rats. Although identical levels of transgene expression in both lines were present, terminally-staged WKY transgenic rats displayed significantly lower final NFT loads than their SHR transgenic counterparts. Interestingly, microglial responses showed a striking difference between transgenic lines. Only 1.6% of microglia in SHR transgenic rats expressed MHCII in spite of having a robust phagocytic phenotype, whereas in WKY transgenic rats, 23.2% of microglia expressed MHCII despite displaying a considerably lower extent of transformation into phagocytic phenotype.
Conclusions: These results show that the immune response represents a pivotal and genetically variable modifying factor that is able to influence vulnerability to neurodegeneration. Therefore, targeted immunomodulation could represent a prospective therapeutic approach to Alzheimer's disease.
Figures



Similar articles
-
Immunomodulation of memory-impairing protein tau in Alzheimer's disease.Neurodegener Dis. 2012;10(1-4):242-5. doi: 10.1159/000333125. Epub 2012 Mar 13. Neurodegener Dis. 2012. PMID: 22433908
-
Truncated tau expression levels determine life span of a rat model of tauopathy without causing neuronal loss or correlating with terminal neurofibrillary tangle load.Eur J Neurosci. 2008 Jul;28(2):239-46. doi: 10.1111/j.1460-9568.2008.06329.x. Eur J Neurosci. 2008. PMID: 18702695
-
Trafficking of immune cells across the blood-brain barrier is modulated by neurofibrillary pathology in tauopathies.PLoS One. 2019 May 23;14(5):e0217216. doi: 10.1371/journal.pone.0217216. eCollection 2019. PLoS One. 2019. PMID: 31120951 Free PMC article.
-
Tau phosphorylation and aggregation as a therapeutic target in tauopathies.CNS Neurol Disord Drug Targets. 2010 Dec;9(6):727-40. doi: 10.2174/187152710793237403. CNS Neurol Disord Drug Targets. 2010. PMID: 20942789 Review.
-
Propagation of Tau Aggregates and Neurodegeneration.Annu Rev Neurosci. 2017 Jul 25;40:189-210. doi: 10.1146/annurev-neuro-072116-031153. Annu Rev Neurosci. 2017. PMID: 28772101 Review.
Cited by
-
Tau Protein and Its Role in Blood-Brain Barrier Dysfunction.Front Mol Neurosci. 2020 Sep 30;13:570045. doi: 10.3389/fnmol.2020.570045. eCollection 2020. Front Mol Neurosci. 2020. PMID: 33100967 Free PMC article. Review.
-
Modeling Alzheimer's disease in transgenic rats.Mol Neurodegener. 2013 Oct 25;8:37. doi: 10.1186/1750-1326-8-37. Mol Neurodegener. 2013. PMID: 24161192 Free PMC article. Review.
-
Genomic background-related activation of microglia and reduced β-amyloidosis in a mouse model of Alzheimer's disease.Eur J Microbiol Immunol (Bp). 2013 Mar 1;3(1):21-27. doi: 10.1556/EuJMI.3.2013.1.3. Eur J Microbiol Immunol (Bp). 2013. PMID: 23814667 Free PMC article.
-
Genetic Background Influences the Propagation of Tau Pathology in Transgenic Rodent Models of Tauopathy.Front Aging Neurosci. 2019 Dec 11;11:343. doi: 10.3389/fnagi.2019.00343. eCollection 2019. Front Aging Neurosci. 2019. PMID: 31920624 Free PMC article.
-
Intersection of pathological tau and microglia at the synapse.Acta Neuropathol Commun. 2019 Jul 5;7(1):109. doi: 10.1186/s40478-019-0754-y. Acta Neuropathol Commun. 2019. PMID: 31277708 Free PMC article. Review.
References
-
- Kivipelto M, Helkala E-L, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Soininnen H, Tuomilehto J, Nissien A. Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Soininnen H, Tuomilehto J, Nissien A. BMJ. 2001;322:1447–14451. doi: 10.1136/bmj.322.7300.1447. - DOI - PMC - PubMed
-
- Stozicka Z, Zilka N, Novak M. Risk and protective factors for sporadic Alzheimer's disease. Acta Virol. 2007;51:205–222. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

