Label-free in vivo optical imaging of functional microcirculations within meninges and cortex in mice
- PMID: 20933005
- PMCID: PMC2993766
- DOI: 10.1016/j.jneumeth.2010.09.021
Label-free in vivo optical imaging of functional microcirculations within meninges and cortex in mice
Abstract
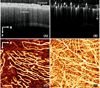
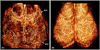
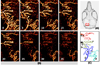
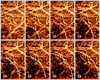
Abnormal microcirculation within meninges is common in many neurological diseases. There is a need for an imaging method that is capable of monitoring dynamic meningeal microcirculations, preferably decoupled from cortical blood flow. Optical microangiography (OMAG) is a recently developed label-free imaging method capable of producing 3D images of dynamic blood perfusion within micro-circulatory tissue beds at an imaging depth up to ∼2 mm, with an unprecedented imaging sensitivity to blood flow at ∼4 μm/s. In this paper, we demonstrate the utility of OMAG in imaging the detailed blood flow distributions, at a capillary level resolution, within the meninges and cortex in mice with the cranium left intact. Using a thrombotic mouse model, we show that the OMAG can yield longitudinal measurements of meningeal vascular responses to the insult and can decouple these responses from those in the cortex, giving valuable information regarding the localized hemodynamics along with the dynamic formation of thrombotic event. The results indicate that OMAG can be a useful tool to study therapeutic strategies in preclinical animal models in order to mitigate various pathologies that are mainly related to the meningeal circulations.
Copyright © 2010 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interests.
Figures

Similar articles
-
Label-free and highly sensitive optical imaging of detailed microcirculation within meninges and cortex in mice with the cranium left intact.J Biomed Opt. 2010 May-Jun;15(3):030510. doi: 10.1117/1.3432654. J Biomed Opt. 2010. PMID: 20614993 Free PMC article.
-
Potential of optical microangiography to monitor cerebral blood perfusion and vascular plasticity following traumatic brain injury in mice in vivo.J Biomed Opt. 2009 Jul-Aug;14(4):040505. doi: 10.1117/1.3207121. J Biomed Opt. 2009. PMID: 19725710 Free PMC article.
-
Optical microangiography provides an ability to monitor responses of cerebral microcirculation to hypoxia and hyperoxia in mice.J Biomed Opt. 2011 Sep;16(9):096019. doi: 10.1117/1.3625238. J Biomed Opt. 2011. PMID: 21950933 Free PMC article.
-
Ultrahigh sensitive optical microangiography reveals depth-resolved microcirculation and its longitudinal response to prolonged ischemic event within skeletal muscles in mice.J Biomed Opt. 2011 Aug;16(8):086004. doi: 10.1117/1.3606565. J Biomed Opt. 2011. PMID: 21895316 Free PMC article.
-
In vivo optical imaging of revascularization after brain trauma in mice.Microvasc Res. 2011 Jan;81(1):73-80. doi: 10.1016/j.mvr.2010.11.003. Epub 2010 Nov 12. Microvasc Res. 2011. PMID: 21075124 Free PMC article.
Cited by
-
Optimization of microCT imaging and blood vessel diameter quantitation of preclinical specimen vasculature with radiopaque polymer injection medium.PLoS One. 2011 Apr 18;6(4):e19099. doi: 10.1371/journal.pone.0019099. PLoS One. 2011. PMID: 21533123 Free PMC article.
-
Responses of peripheral blood flow to acute hypoxia and hyperoxia as measured by optical microangiography.PLoS One. 2011;6(10):e26802. doi: 10.1371/journal.pone.0026802. Epub 2011 Oct 25. PLoS One. 2011. PMID: 22046363 Free PMC article.
-
Optical coherence tomography based angiography [Invited].Biomed Opt Express. 2017 Jan 24;8(2):1056-1082. doi: 10.1364/BOE.8.001056. eCollection 2017 Feb 1. Biomed Opt Express. 2017. PMID: 28271003 Free PMC article. Review.
-
Adaptive contour-tracking to aid wide-field swept-source optical coherence tomography imaging of large objects with uneven surface topology.Biomed Opt Express. 2024 Jul 29;15(8):4891-4908. doi: 10.1364/BOE.533399. eCollection 2024 Aug 1. Biomed Opt Express. 2024. PMID: 39347000 Free PMC article.
-
Swept-source optical coherence tomography powered by a 1.3-μm vertical cavity surface emitting laser enables 2.3-mm-deep brain imaging in mice in vivo.J Biomed Opt. 2015 Oct;20(10):106004. doi: 10.1117/1.JBO.20.10.106004. J Biomed Opt. 2015. PMID: 26447860 Free PMC article.
References
-
- Bartholomaus I, Kawakami N, Odoardi F, C S, Miljkovic D, Ellwart JW, Klinkert WEF, Flugel-Koch C, Issekutz TB, Wekerle H, Flugel A. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462:94–98. - PubMed
-
- Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med. 2002;8:136–142. - PubMed
-
- Fercher AF, Drexler W, Hitzenberger CK, Lasser T. Optical Coherence Tomography - Principles and Applications. Rep Prog Phys. 2003;66:239–303.
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL093140-04/HL/NHLBI NIH HHS/United States
- R01 HL093140/HL/NHLBI NIH HHS/United States
- R01 DC010201-03/DC/NIDCD NIH HHS/United States
- R01 EB009682-02/EB/NIBIB NIH HHS/United States
- R01 HL093140-03/HL/NHLBI NIH HHS/United States
- R01 DC010201-02/DC/NIDCD NIH HHS/United States
- R01 EB009682-01/EB/NIBIB NIH HHS/United States
- R01 EB009682-03/EB/NIBIB NIH HHS/United States
- R01 DC010201-01/DC/NIDCD NIH HHS/United States
- R01 HL093140-02/HL/NHLBI NIH HHS/United States
- R01 HL093140-01/HL/NHLBI NIH HHS/United States
- R01 EB009682/EB/NIBIB NIH HHS/United States
- R01 DC010201/DC/NIDCD NIH HHS/United States
- R01 HL093140-01S1/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources

