Novel anti-carbohydrate antibodies reveal the cooperative function of sulfated N- and O-glycans in lymphocyte homing
- PMID: 20929857
- PMCID: PMC3003387
- DOI: 10.1074/jbc.M110.167296
Novel anti-carbohydrate antibodies reveal the cooperative function of sulfated N- and O-glycans in lymphocyte homing
Abstract
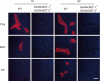
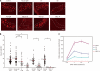
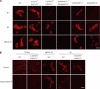
Cell surface glycans play pivotal roles in immune cell trafficking and immunity. Here we present an efficient method for generating anti-carbohydrate monoclonal antibodies (mAbs) using gene-targeted mice and describe critical glycans in lymphocyte homing. We immunized sulfotransferase GlcNAc6ST-1 and GlcNAc6ST-2 doubly deficient mice with sulfotransferase-overexpressing Chinese hamster ovary cells and generated two mAbs, termed S1 and S2. Both S1 and S2 bound high endothelial venules (HEVs) in the lymphoid organs of humans and wild-type mice, but not in those of doubly deficient mice. Glycan array analysis indicated that both S1 and S2 specifically bound 6-sulfo sialyl Lewis X and its defucosylated structure. Interestingly, S2 inhibited lymphocyte homing to peripheral lymph nodes by 95%, whereas S1 inhibited it by only 25%. S2 also significantly inhibited contact hypersensitivity responses and L-selectin-dependent leukocyte adhesion to HEVs. Immunohistochemical and Western blot analyses indicated that S1 preferentially bound sulfated O-glycans, whereas S2 bound both sulfated N- and O-glycans in HEVs. Furthermore, S2 strongly inhibited the N-glycan-dependent residual lymphocyte homing in mutant mice lacking sulfated O-glycans, indicating the importance of both sulfated N- and O-glycans in lymphocyte homing. Thus, the two mAbs generated by a novel method revealed the cooperative function of sulfated N- and O-glycans in lymphocyte homing and immune surveillance.
Figures


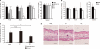

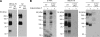
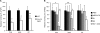

Similar articles
-
Significant decrease in alpha1,3-linked fucose in association with increase in 6-sulfated N-acetylglucosamine in peripheral lymph node addressin of FucT-VII-deficient mice exhibiting diminished lymphocyte homing.Glycobiology. 2007 Mar;17(3):277-93. doi: 10.1093/glycob/cwl077. Epub 2006 Dec 15. Glycobiology. 2007. PMID: 17172261
-
N-acetylglucosamine-6-O-sulfotransferases 1 and 2 cooperatively control lymphocyte homing through L-selectin ligand biosynthesis in high endothelial venules.Nat Immunol. 2005 Nov;6(11):1096-104. doi: 10.1038/ni1259. Epub 2005 Oct 9. Nat Immunol. 2005. PMID: 16227985
-
Sulfated glycans control lymphocyte homing.Ann N Y Acad Sci. 2012 Apr;1253:112-21. doi: 10.1111/j.1749-6632.2011.06356.x. Epub 2012 Jan 30. Ann N Y Acad Sci. 2012. PMID: 22288521 Review.
-
Functional contributions of N- and O-glycans to L-selectin ligands in murine and human lymphoid organs.Am J Pathol. 2011 Jan;178(1):423-33. doi: 10.1016/j.ajpath.2010.11.009. Epub 2010 Dec 23. Am J Pathol. 2011. PMID: 21224079 Free PMC article.
-
Roles of sulfated glycans in lymphocyte homing.Biol Pharm Bull. 2006 Dec;29(12):2343-9. doi: 10.1248/bpb.29.2343. Biol Pharm Bull. 2006. PMID: 17142960 Review.
Cited by
-
KSGal6ST generates galactose-6-O-sulfate in high endothelial venules but does not contribute to L-selectin-dependent lymphocyte homing.Glycobiology. 2013 Mar;23(3):381-94. doi: 10.1093/glycob/cws166. Epub 2012 Dec 18. Glycobiology. 2013. PMID: 23254996 Free PMC article.
-
Chemoenzymatic Synthesis of Sulfated N-Glycans Recognized by Siglecs and Other Glycan-Binding Proteins.JACS Au. 2024 Jul 26;4(8):2966-2978. doi: 10.1021/jacsau.4c00307. eCollection 2024 Aug 26. JACS Au. 2024. PMID: 39211606 Free PMC article.
-
A sialylated glycan microarray reveals novel interactions of modified sialic acids with proteins and viruses.J Biol Chem. 2011 Sep 9;286(36):31610-22. doi: 10.1074/jbc.M111.274217. Epub 2011 Jul 12. J Biol Chem. 2011. PMID: 21757734 Free PMC article.
-
Modifications of glycans: biological significance and therapeutic opportunities.ACS Chem Biol. 2012 Jan 20;7(1):31-43. doi: 10.1021/cb2004466. Epub 2012 Jan 11. ACS Chem Biol. 2012. PMID: 22195988 Free PMC article. Review.
-
High endothelial venules (HEVs) in immunity, inflammation and cancer.Angiogenesis. 2021 Nov;24(4):719-753. doi: 10.1007/s10456-021-09792-8. Epub 2021 May 6. Angiogenesis. 2021. PMID: 33956259 Free PMC article. Review.
References
-
- Varki A., Cummings R. D., Esko J. D., Freeze H. H., Stanley P., Bertozzi C. R., Hart G. W., Etzler M. E. (eds) (2009) Essentials of Glycobiology, 2nd Ed., Cold Spring Harbor Laboratory, Cold Spring Harbor, NY - PubMed
-
- von Andrian U. H., Mempel T. R. (2003) Nat. Rev. Immunol. 3, 867–878 - PubMed
-
- Butcher E. C., Picker L. J. (1996) Science 272, 60–66 - PubMed
-
- Rosen S. D. (2004) Annu. Rev. Immunol. 22, 129–156 - PubMed
-
- Kawashima H. (2006) Biol. Pharm. Bull. 29, 2343–2349 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

