IL-2 and IL-4 stimulate MEK1 expression and contribute to T cell resistance against suppression by TGF-beta and IL-10 in asthma
- PMID: 20926789
- PMCID: PMC3367768
- DOI: 10.4049/jimmunol.1000690
IL-2 and IL-4 stimulate MEK1 expression and contribute to T cell resistance against suppression by TGF-beta and IL-10 in asthma
Abstract
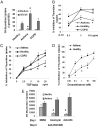
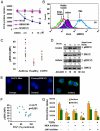
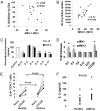
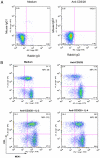
The T cell-driven airway inflammation in chronic asthma is uninhibited and sustained. We examined the resistance of T cells from asthmatic patients against suppression by TGF-β, IL-10 and glucocorticoids and explored its signaling mechanism. CD4(+)CD25(-) T cells from allergic asthmatic subjects demonstrated increased TCR-stimulated proliferation as compared with healthy and chronic obstructive pulmonary disease controls. This proliferation was resistant to inhibition by TGF-β, IL-10, and dexamethasone and to anergy induction. CD4 T cells from asthmatic patients, but not chronic obstructive pulmonary disease, allergic rhinitis, and healthy subjects, showed increased expression of MEK1, heightened phosphorylation of ERK1/2, and increased levels of c-Fos. IL-2 and IL-4 stimulated the expression of MEK1 and c-Fos and induced T cell resistance. The inhibition of MEK1 reversed, whereas induced expression of c-Fos and JunB promoted T cell resistance against TGF-β- and IL-10-mediated suppression. We have uncovered an IL-2- and IL-4-driven MEK1 induction mechanism that results in heightened ERK1/2 activation in asthmatic T cells and make them resistant to certain inhibitory mechanisms.
Figures

Similar articles
-
Human anergic/suppressive CD4(+)CD25(+) T cells: a highly differentiated and apoptosis-prone population.Eur J Immunol. 2001 Apr;31(4):1122-31. doi: 10.1002/1521-4141(200104)31:4<1122::aid-immu1122>3.0.co;2-p. Eur J Immunol. 2001. PMID: 11298337
-
Inhibition of human allergic T-helper type 2 immune responses by induced regulatory T cells requires the combination of interleukin-10-treated dendritic cells and transforming growth factor-beta for their induction.Clin Exp Allergy. 2006 Dec;36(12):1546-55. doi: 10.1111/j.1365-2222.2006.02601.x. Clin Exp Allergy. 2006. PMID: 17177678
-
β-arrestin2 stimulates interleukin-17 production and expression of CD4+ T lymphocytes in a murine asthma model.Iran J Allergy Asthma Immunol. 2011 Sep;10(3):171-82. Iran J Allergy Asthma Immunol. 2011. PMID: 21891823
-
A comparative study of the effects of inhibitory cytokines on human natural killer cells and the mechanistic features of transforming growth factor-beta.Cell Immunol. 2014 Jul;290(1):52-61. doi: 10.1016/j.cellimm.2014.05.001. Epub 2014 May 13. Cell Immunol. 2014. PMID: 24879062
-
Regulatory T cells in asthma.Immunity. 2009 Sep 18;31(3):438-49. doi: 10.1016/j.immuni.2009.08.007. Immunity. 2009. PMID: 19766086 Free PMC article. Review.
Cited by
-
Mechanisms and functions of SUMOylation in health and disease: a review focusing on immune cells.J Biomed Sci. 2024 Jan 27;31(1):16. doi: 10.1186/s12929-024-01003-y. J Biomed Sci. 2024. PMID: 38280996 Free PMC article. Review.
-
Genotype-dependent effects of TGF-β1 on mast cell function: targeting the Stat5 pathway.J Immunol. 2013 Nov 1;191(9):4505-13. doi: 10.4049/jimmunol.1202723. Epub 2013 Sep 25. J Immunol. 2013. PMID: 24068671 Free PMC article.
-
Nuclear translocation of MEK1 triggers a complex T cell response through the corepressor silencing mediator of retinoid and thyroid hormone receptor.J Immunol. 2013 Jan 1;190(1):159-67. doi: 10.4049/jimmunol.1201657. Epub 2012 Dec 5. J Immunol. 2013. PMID: 23225884 Free PMC article.
-
TGF-β induces the expression of the adaptor Ndfip1 to silence IL-4 production during iTreg cell differentiation.Nat Immunol. 2011 Nov 13;13(1):77-85. doi: 10.1038/ni.2154. Nat Immunol. 2011. PMID: 22080920 Free PMC article.
-
Emerging Regulatory Roles of Dual-Specificity Phosphatases in Inflammatory Airway Disease.Int J Mol Sci. 2019 Feb 5;20(3):678. doi: 10.3390/ijms20030678. Int J Mol Sci. 2019. PMID: 30764493 Free PMC article. Review.
References
-
- Robinson DS, Hamid Q, Jacobson M, Ying S, Kay AB, Durham SR. Evidence for Th2-type T helper cell control of allergic disease in vivo. Springer Semin. Immunopathol. 1993;15:17–27. - PubMed
-
- Robinson D, Hamid Q, Bentley A, Ying S, Kay AB, Durham SR. Activation of CD4+ T cells, increased TH2-type cytokine mRNA expression, and eosinophil recruitment in bronchoalveolar lavage after allergen inhalation challenge in patients with atopic asthma. J. Allergy Clin. Immunol. 1993;92:313–324. - PubMed
-
- O’Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat. Med. 2004;10:801–805. - PubMed
-
- Shevach EM. Regulatory T cells in autoimmmunity. Annu. Rev. Immunol. 2000;18:423–449. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

