Dual functions of Nbs1 in the repair of DNA breaks and proliferation ensure proper V(D)J recombination and T-cell development
- PMID: 20921278
- PMCID: PMC2976431
- DOI: 10.1128/MCB.00917-10
Dual functions of Nbs1 in the repair of DNA breaks and proliferation ensure proper V(D)J recombination and T-cell development
Abstract
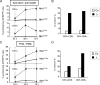
Immunodeficiency and lymphoid malignancy are hallmarks of the human disease Nijmegen breakage syndrome (NBS; OMIM 251260), which is caused by NBS1 mutations. Although NBS1 has been shown to bind to the T-cell receptor alpha (TCRα) locus, its role in TCRβ rearrangement is unclear. Hypomorphic mutations of Nbs1 in mice and patients result in relatively mild T-cell deficiencies, raising the question of whether the truncated Nbs1 protein might have clouded a certain function of NBS1 in T-cell development. Here we show that the deletion of the entire Nbs1 protein in T-cell precursors (Nbs1(T-del)) results in severe lymphopenia and a hindrance to the double-negative 3 (DN3)-to-DN4 transition in early T-cell development, due to abnormal TCRβ coding and signal joints as well as the functions of Nbs1 in T-cell expansion. Chromatin immunoprecipitation (ChIP) analysis of the TCR loci reveals that Nbs1 depletion compromises the loading of Mre11/Rad50 to V(D)J-generated DNA double-strand breaks (DSBs) and thereby affects resection of DNA termini and chromatin conformation of the postcleavage complex. Although a p53 deficiency relieves the DN3→DN4 transition block, neither a p53 deficiency nor ectopic expression of TCRαβ rescues the major T-cell loss in Nbs1(T-del) mice. All together, these results demonstrate that Nbs1's functions in both repair of V(D)J-generated DSBs and proliferation are essential for T-cell development.
Figures
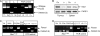

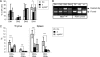



Similar articles
-
NBS1 cooperates with homologous recombination to counteract chromosome breakage during replication.DNA Repair (Amst). 2009 Dec 3;8(12):1363-70. doi: 10.1016/j.dnarep.2009.09.002. Epub 2009 Sep 25. DNA Repair (Amst). 2009. PMID: 19782649 Free PMC article.
-
Loss of juxtaposition of RAG-induced immunoglobulin DNA ends is implicated in the precursor B-cell differentiation defect in NBS patients.Blood. 2010 Jun 10;115(23):4770-7. doi: 10.1182/blood-2009-10-250514. Epub 2010 Apr 8. Blood. 2010. PMID: 20378756
-
Normal V(D)J recombination in cells from patients with Nijmegen breakage syndrome.Mol Immunol. 2000 Oct;37(15):915-29. doi: 10.1016/s0161-5890(01)00008-6. Mol Immunol. 2000. PMID: 11282395
-
Nijmegen breakage syndrome gene, NBS1, and molecular links to factors for genome stability.Oncogene. 2002 Dec 16;21(58):8967-80. doi: 10.1038/sj.onc.1206136. Oncogene. 2002. PMID: 12483513 Review.
-
Nijmegen breakage syndrome and functions of the responsible protein, NBS1.Genome Dyn. 2006;1:191-205. doi: 10.1159/000092508. Genome Dyn. 2006. PMID: 18724061 Review.
Cited by
-
PID comes full circle: applications of V(D)J recombination excision circles in research, diagnostics and newborn screening of primary immunodeficiency disorders.Front Immunol. 2011 May 4;2:12. doi: 10.3389/fimmu.2011.00012. eCollection 2011. Front Immunol. 2011. PMID: 22566803 Free PMC article.
-
Telomeric epigenetic response mediated by Gadd45a regulates stem cell aging and lifespan.EMBO Rep. 2018 Oct;19(10):e45494. doi: 10.15252/embr.201745494. Epub 2018 Aug 20. EMBO Rep. 2018. PMID: 30126922 Free PMC article.
-
The MRE11 complex: starting from the ends.Nat Rev Mol Cell Biol. 2011 Feb;12(2):90-103. doi: 10.1038/nrm3047. Nat Rev Mol Cell Biol. 2011. PMID: 21252998 Free PMC article. Review.
-
The response to and repair of RAG-mediated DNA double-strand breaks.Annu Rev Immunol. 2012;30:175-202. doi: 10.1146/annurev-immunol-030409-101320. Epub 2012 Jan 3. Annu Rev Immunol. 2012. PMID: 22224778 Free PMC article. Review.
-
Defining ATM-Independent Functions of the Mre11 Complex with a Novel Mouse Model.Mol Cancer Res. 2016 Feb;14(2):185-95. doi: 10.1158/1541-7786.MCR-15-0281. Epub 2015 Nov 4. Mol Cancer Res. 2016. PMID: 26538284 Free PMC article.
References
-
- Barlow, C., S. Hirotsune, R. Paylor, M. Liyanage, M. Eckhaus, F. Collins, Y. Shiloh, J. N. Crawley, T. Ried, D. Tagle, and A. Wynshaw-Boris. 1996. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86:159-171. - PubMed
-
- Bassing, C. H., W. Swat, and F. W. Alt. 2002. The mechanism and regulation of chromosomal V(D)J. recombination. Cell 109:S45-S55. - PubMed
-
- Bird, A. W., D. Y. Yu, M. G. Pray-Grant, Q. Qui, K. E. Harmon, P. C. Megee, P. A. Grant, M. M. Smith, and M. F. Christman. 2002. Acetylation of histone H4 by Esa1 is required for DNA double-strand break repair. Nature 419:411-415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
