Oscillatory shear stress induces mitochondrial superoxide production: implication of NADPH oxidase and c-Jun NH2-terminal kinase signaling
- PMID: 20919940
- PMCID: PMC3144427
- DOI: 10.1089/ars.2010.3645
Oscillatory shear stress induces mitochondrial superoxide production: implication of NADPH oxidase and c-Jun NH2-terminal kinase signaling
Abstract
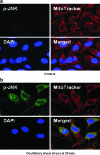
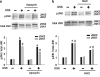
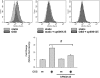
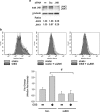
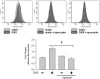
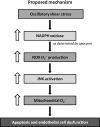
Fluid shear stress is intimately linked with vascular oxidative stress and atherosclerosis. We posited that atherogenic oscillatory shear stress (OSS) induced mitochondrial superoxide (mtO2•-) production via NADPH oxidase and c-Jun NH(2)-terminal kinase (JNK-1 and JNK-2) signaling. In bovine aortic endothelial cells, OSS (±3 dyn/cm2) induced JNK activation, which peaked at 1 h, accompanied by an increase in fluorescein isothiocyanate-conjugated JNK fluorescent and MitoSOX Red (specific for mtO2•- production) intensities. Pretreatment with apocynin (NADPH oxidase inhibitor) or N-acetyl cysteine (antioxidant) significantly attenuated OSS-induced JNK activation. Apocynin further reduced OSS-mediated dihydroethidium and MitoSOX Red intensities specific for cytosolic O2•- and mtO2•- production, respectively. As a corollary, transfecting bovine aortic endothelial cells with JNK siRNA (siJNK) and pretreating with SP600125 (JNK inhibitor) significantly attenuated OSS-mediated mtO2•- production. Immunohistochemistry on explants of human coronary arteries further revealed prominent phosphorylated JNK staining in OSS-exposed regions. These findings indicate that OSS induces mtO2•- production via NADPH oxidase and JNK activation relevant for vascular oxidative stress.
Figures



Similar articles
-
Disturbed Flow Induces Autophagy, but Impairs Autophagic Flux to Perturb Mitochondrial Homeostasis.Antioxid Redox Signal. 2015 Nov 20;23(15):1207-19. doi: 10.1089/ars.2014.5896. Epub 2015 Jun 29. Antioxid Redox Signal. 2015. PMID: 26120766 Free PMC article.
-
Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress.Am J Physiol Heart Circ Physiol. 2003 Dec;285(6):H2290-7. doi: 10.1152/ajpheart.00515.2003. Epub 2003 Sep 4. Am J Physiol Heart Circ Physiol. 2003. PMID: 12958034
-
Differential Roles of Protein Complexes NOX1-NOXO1 and NOX2-p47phox in Mediating Endothelial Redox Responses to Oscillatory and Unidirectional Laminar Shear Stress.J Biol Chem. 2016 Apr 15;291(16):8653-62. doi: 10.1074/jbc.M115.713149. Epub 2016 Jan 29. J Biol Chem. 2016. PMID: 26826128 Free PMC article.
-
Shear stress influences spatial variations in vascular Mn-SOD expression: implication for LDL nitration.Am J Physiol Cell Physiol. 2008 Jun;294(6):C1576-85. doi: 10.1152/ajpcell.00518.2007. Epub 2008 Apr 23. Am J Physiol Cell Physiol. 2008. PMID: 18434620 Free PMC article.
-
Flow shear stress and atherosclerosis: a matter of site specificity.Antioxid Redox Signal. 2011 Sep 1;15(5):1405-14. doi: 10.1089/ars.2010.3679. Epub 2011 Apr 8. Antioxid Redox Signal. 2011. PMID: 21050140 Free PMC article. Review.
Cited by
-
Laminar flow activation of ERK5 protein in vascular endothelium leads to atheroprotective effect via NF-E2-related factor 2 (Nrf2) activation.J Biol Chem. 2012 Nov 23;287(48):40722-31. doi: 10.1074/jbc.M112.381509. Epub 2012 Oct 5. J Biol Chem. 2012. PMID: 23043106 Free PMC article.
-
Oscillatory cerebral blood flow is associated with impaired neurocognition and functional hyperemia in postural tachycardia syndrome during graded tilt.Hypertension. 2015 Mar;65(3):636-43. doi: 10.1161/HYPERTENSIONAHA.114.04576. Epub 2014 Dec 15. Hypertension. 2015. PMID: 25510829 Free PMC article.
-
Bioreactor Expansion Reconfigures Metabolism and Extracellular Vesicle Biogenesis of Human Adipose-derived Stem Cells In Vitro.Biochem Eng J. 2022 Dec 15;188:108711. doi: 10.1016/j.bej.2022.108711. Epub 2022 Nov 2. Biochem Eng J. 2022. PMID: 36540623 Free PMC article.
-
Laminar Flow Inhibits ER Stress-Induced Endothelial Apoptosis through PI3K/Akt-Dependent Signaling Pathway.Mol Cells. 2018 Nov 30;41(11):964-970. doi: 10.14348/molcells.2018.0111. Epub 2018 Nov 1. Mol Cells. 2018. PMID: 30396238 Free PMC article.
-
Antioxidant supplements and endurance exercise: Current evidence and mechanistic insights.Redox Biol. 2020 Aug;35:101471. doi: 10.1016/j.redox.2020.101471. Epub 2020 Feb 20. Redox Biol. 2020. PMID: 32127289 Free PMC article. Review.
References
-
- Aoki H. Kang PM. Hampe J. Yoshimura K. Noma T. Matsuzaki M. Izumo S. Direct activation of mitochondrial apoptosis machinery by c-Jun N-terminal kinase in adult cardiac myocytes. J Biol Chem. 2002;277:10244–10250. - PubMed
-
- Arita Y. Harkness SH. Kazzaz JA. Koo HC. Joseph A. Melendez JA. Davis JM. Chander A. Li Y. Mitochondrial localization of catalase provides optimal protection from H2O2-induced cell death in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L978–L986. - PubMed
-
- Bochkov V. Kadl A. Huber J. Gruber F. Binder B. Leitinger N. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature. 2002;419:77–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

