The ATM cofactor ATMIN protects against oxidative stress and accumulation of DNA damage in the aging brain
- PMID: 20889973
- PMCID: PMC2992286
- DOI: 10.1074/jbc.M110.145896
The ATM cofactor ATMIN protects against oxidative stress and accumulation of DNA damage in the aging brain
Abstract
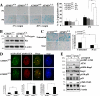
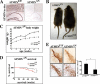
Progressive accumulation of DNA damage is causally involved in cellular senescence and organismal aging. The DNA damage kinase ATM plays a central role in maintaining genomic stability. ATM mutations cause the genetic disorder ataxia telangiectasia, which is primarily characterized by progressive neurodegeneration and cancer susceptibility. Although the importance of ATM function to protect against oxidative DNA damage and during aging is well described, the mechanism of ATM activation by these stimuli is not known. Here we identify ATM interactor (ATMIN) as an essential component of the ATM signaling pathway in response to oxidative stress and aging. Embryos lacking ATMIN (atmin(Δ/Δ)) died in utero and showed increased numbers of cells positive for phosphorylated histone H2aX, indicative of increased DNA damage. atmin(Δ/Δ) mouse embryonic fibroblasts accumulated DNA damage and prematurely entered senescence when cultured at atmospheric oxygen levels (20%), but this defect was rescued by addition of an antioxidant and also by culturing cells at physiological oxygen levels (3%). In response to acute oxidative stress, atmin(Δ/Δ) mouse embryonic fibroblasts showed slightly lower levels of ATM phosphorylation and reduced ATM substrate phosphorylation. Conditional deletion of ATMIN in the murine nervous system (atmin(ΔN)) resulted in reduced numbers of dopaminergic neurons, as does ATM deficiency. ATM activity was observed in old, but not in young, control mice, but aging-induced ATM signaling was impaired by ATMIN deficiency. Consequently, old atmin(ΔN) mice showed accumulation of DNA damage in the cortex accompanied by gliosis, resulting in increased mortality of aging mutant mice. These results suggest that ATMIN mediates ATM activation by oxidative stress, and thereby ATMIN protects the aging brain by preventing accumulation of DNA damage.
Figures



Similar articles
-
Competition between NBS1 and ATMIN controls ATM signaling pathway choice.Cell Rep. 2012 Dec 27;2(6):1498-504. doi: 10.1016/j.celrep.2012.11.002. Epub 2012 Dec 6. Cell Rep. 2012. PMID: 23219553
-
ATMIN defines an NBS1-independent pathway of ATM signalling.EMBO J. 2007 Jun 20;26(12):2933-41. doi: 10.1038/sj.emboj.7601733. Epub 2007 May 24. EMBO J. 2007. PMID: 17525732 Free PMC article.
-
Ataxia telangiectasia mutated (ATM)-mediated DNA damage response in oxidative stress-induced vascular endothelial cell senescence.J Biol Chem. 2010 Sep 17;285(38):29662-70. doi: 10.1074/jbc.M110.125138. Epub 2010 Jul 16. J Biol Chem. 2010. PMID: 20639198 Free PMC article.
-
ATMINistrating ATM signalling: regulation of ATM by ATMIN.Cell Cycle. 2008 Nov 15;7(22):3483-6. doi: 10.4161/cc.7.22.7044. Epub 2008 Nov 18. Cell Cycle. 2008. PMID: 19001856 Review.
-
Constitutive histone H2AX phosphorylation and ATM activation, the reporters of DNA damage by endogenous oxidants.Cell Cycle. 2006 Sep;5(17):1940-5. doi: 10.4161/cc.5.17.3191. Epub 2006 Sep 1. Cell Cycle. 2006. PMID: 16940754 Free PMC article. Review.
Cited by
-
Neuronal c-Jun is required for successful axonal regeneration, but the effects of phosphorylation of its N-terminus are moderate.J Neurochem. 2012 May;121(4):607-18. doi: 10.1111/j.1471-4159.2012.07706.x. Epub 2012 Mar 21. J Neurochem. 2012. PMID: 22372722 Free PMC article.
-
Heat-induced increases in body temperature in lactating dairy cows: impact on the cumulus and granulosa cell transcriptome of the periovulatory follicle.J Anim Sci. 2022 Jul 1;100(7):skac121. doi: 10.1093/jas/skac121. J Anim Sci. 2022. PMID: 35772768 Free PMC article.
-
Spatiotemporal dynamics of γH2AX in the mouse brain after acute irradiation at different postnatal days with special reference to the dentate gyrus of the hippocampus.Aging (Albany NY). 2021 Jun 23;13(12):15815-15832. doi: 10.18632/aging.203202. Epub 2021 Jun 23. Aging (Albany NY). 2021. PMID: 34162763 Free PMC article.
-
Aging Neurovascular Unit and Potential Role of DNA Damage and Repair in Combating Vascular and Neurodegenerative Disorders.Front Neurosci. 2019 Aug 8;13:778. doi: 10.3389/fnins.2019.00778. eCollection 2019. Front Neurosci. 2019. PMID: 31440124 Free PMC article. Review.
-
ATMIN is required for maintenance of genomic stability and suppression of B cell lymphoma.Cancer Cell. 2011 May 17;19(5):587-600. doi: 10.1016/j.ccr.2011.03.022. Cancer Cell. 2011. PMID: 21575860 Free PMC article.
References
-
- Sedelnikova O. A., Horikawa I., Zimonjic D. B., Popescu N. C., Bonner W. M., Barrett J. C. (2004) Nat. Cell Biol. 6, 168–170 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

