Brain-penetrating tumor necrosis factor decoy receptor in the mouse
- PMID: 20884844
- PMCID: PMC3014268
- DOI: 10.1124/dmd.110.036012
Brain-penetrating tumor necrosis factor decoy receptor in the mouse
Abstract
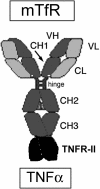
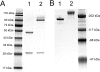
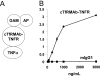
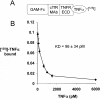
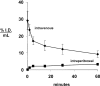
Biologic tumor necrosis factor inhibitors (TNFIs) include TNF decoy receptors (TNFRs). TNFα plays a pathologic role in both acute and chronic brain disease. However, biologic TNFIs cannot be developed as brain therapeutics because these large molecule drugs do not cross the blood-brain barrier (BBB). To enable penetration of the brain via receptor-mediated transport, the human TNFR type II was re-engineered as an IgG fusion protein, where the IgG part is a chimeric monoclonal antibody (MAb) against the mouse transferrin receptor (TfR), and this fusion protein is designated cTfRMAb-TNFR. The cTfRMAb part of the fusion protein acts as a molecular Trojan horse to ferry the TNFR across the BBB via transport on the endogenous BBB TfR. cTfRMAb-TNFR was expressed by stably transfected Chinese hamster ovary cells and purified by affinity chromatography to homogeneity on electrophoretic gels. The fusion protein reacted with antibodies to both mouse IgG and the human TNFR and bound TNFα with high affinity (K(d) = 96 ± 34 pM). cTfRMAb-TNFR was rapidly transported into mouse brain in vivo after intravenous administration, and the brain uptake of the fusion protein was 2.8 ± 0.5% of injected dose per gram of brain, which is >45-fold higher than the brain uptake of an IgG that does not recognize the mouse TfR. This new IgG-TNFR fusion protein can be tested in mouse models of brain diseases in which TNFα plays a pathologic role.
Figures
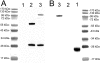
Similar articles
-
Brain protection from stroke with intravenous TNFα decoy receptor-Trojan horse fusion protein.J Cereb Blood Flow Metab. 2012 Oct;32(10):1933-8. doi: 10.1038/jcbfm.2012.97. Epub 2012 Jun 20. J Cereb Blood Flow Metab. 2012. PMID: 22714051 Free PMC article.
-
Neuroprotection with a brain-penetrating biologic tumor necrosis factor inhibitor.J Pharmacol Exp Ther. 2011 Nov;339(2):618-23. doi: 10.1124/jpet.111.185876. Epub 2011 Aug 10. J Pharmacol Exp Ther. 2011. PMID: 21831964 Free PMC article.
-
Selective targeting of a TNFR decoy receptor pharmaceutical to the primate brain as a receptor-specific IgG fusion protein.J Biotechnol. 2010 Mar;146(1-2):84-91. doi: 10.1016/j.jbiotec.2010.01.011. Epub 2010 Jan 25. J Biotechnol. 2010. PMID: 20100527 Free PMC article.
-
Blood-brain barrier drug delivery of IgG fusion proteins with a transferrin receptor monoclonal antibody.Expert Opin Drug Deliv. 2015 Feb;12(2):207-22. doi: 10.1517/17425247.2014.952627. Epub 2014 Aug 20. Expert Opin Drug Deliv. 2015. PMID: 25138991 Review.
-
Reengineering biopharmaceuticals for targeted delivery across the blood-brain barrier.Methods Enzymol. 2012;503:269-92. doi: 10.1016/B978-0-12-396962-0.00011-2. Methods Enzymol. 2012. PMID: 22230573 Review.
Cited by
-
Agile delivery of protein therapeutics to CNS.J Control Release. 2014 Sep 28;190:637-63. doi: 10.1016/j.jconrel.2014.06.017. Epub 2014 Jun 21. J Control Release. 2014. PMID: 24956489 Free PMC article. Review.
-
Brain protection from stroke with intravenous TNFα decoy receptor-Trojan horse fusion protein.J Cereb Blood Flow Metab. 2012 Oct;32(10):1933-8. doi: 10.1038/jcbfm.2012.97. Epub 2012 Jun 20. J Cereb Blood Flow Metab. 2012. PMID: 22714051 Free PMC article.
-
New hope for survivors of stroke and traumatic brain injury.CNS Drugs. 2012 Dec;26(12):1071-2. doi: 10.1007/s40263-012-0014-1. CNS Drugs. 2012. PMID: 23100197 No abstract available.
-
Tumor necrosis factor-α synthesis inhibitor, 3,6'-dithiothalidomide, reverses behavioral impairments induced by minimal traumatic brain injury in mice.J Neurochem. 2011 Sep;118(6):1032-42. doi: 10.1111/j.1471-4159.2011.07377.x. Epub 2011 Aug 5. J Neurochem. 2011. PMID: 21740439 Free PMC article.
-
Receptor-mediated abeta amyloid antibody targeting to Alzheimer's disease mouse brain.Mol Pharm. 2011 Feb 7;8(1):280-5. doi: 10.1021/mp1003515. Epub 2010 Dec 21. Mol Pharm. 2011. PMID: 21141969 Free PMC article.
References
-
- Banner DW, D'Arcy A, Janes W, Gentz R, Schoenfeld HJ, Broger C, Loetscher H, Lesslauer W. (1993) Crystal structure of the soluble human 55 kd TNF receptor-human TNF beta complex: implications for TNF receptor activation. Cell 73:431–445 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

