Lesions in posterior parietal area 5 in monkeys result in rapid behavioral and cortical plasticity
- PMID: 20881111
- PMCID: PMC3432266
- DOI: 10.1523/JNEUROSCI.1806-10.2010
Lesions in posterior parietal area 5 in monkeys result in rapid behavioral and cortical plasticity
Abstract
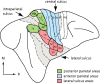
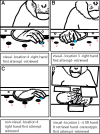
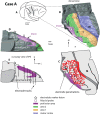
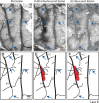
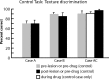
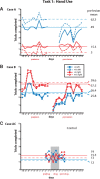
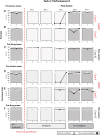
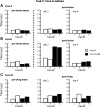
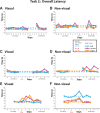
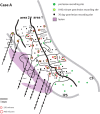
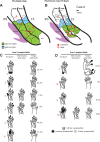
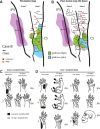
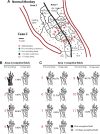
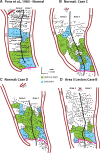
We examined the effects of focal lesions of posterior parietal area 5 in macaque monkeys on bimanual behavior performed with and without visual guidance. The animals were trained on two reaching tasks and one tactile texture discrimination task. Task 1 simply involved reaching toward and grasping a reward from one of five well positions. Task 2 required the monkey to use both hands simultaneously to obtain a reward. The tactile texture discrimination task required the monkey to signal the roughness of a passively delivered texture using its jaw. After lesions to area 5, the monkeys showed a decrease in hand use for tasks 1 and 2 and an inability to perform task 2 in specific locations in visual space. These deficits recovered within several days. No deficits were observed in the tactile texture discrimination task or in an analgesic control monkey. Electrophysiological recordings made just before the lesion, immediately after the lesion, and 2 months after the lesion demonstrated that cortical areas just rostral to the lesioned area 5, and areas 1 and 2, were topographically reorganized and that receptive fields for neurons in these fields changed location on the body surface. These cortical map changes are correlative and may, in part, contribute to the rapid behavioral recovery observed. The mechanism for such rapid changes may be the unmasking of existing divergent and convergent thalamocortical connections that are part of the normal cortical circuitry.
Figures


Similar articles
-
Differential effects of the mode of touch, active and passive, on experience-driven plasticity in the S1 cutaneous digit representation of adult macaque monkeys.J Neurophysiol. 2020 Mar 1;123(3):1072-1089. doi: 10.1152/jn.00014.2019. Epub 2020 Feb 5. J Neurophysiol. 2020. PMID: 32023143
-
Topographic reorganization of the hand representation in cortical area 3b owl monkeys trained in a frequency-discrimination task.J Neurophysiol. 1992 May;67(5):1031-56. doi: 10.1152/jn.1992.67.5.1031. J Neurophysiol. 1992. PMID: 1597696
-
Neurophysiology of prehension. I. Posterior parietal cortex and object-oriented hand behaviors.J Neurophysiol. 2007 Jan;97(1):387-406. doi: 10.1152/jn.00558.2006. Epub 2006 Sep 13. J Neurophysiol. 2007. PMID: 16971679 Free PMC article.
-
Parietofrontal circuits for action and space perception in the macaque monkey.Neuroimage. 2001 Jul;14(1 Pt 2):S27-32. doi: 10.1006/nimg.2001.0835. Neuroimage. 2001. PMID: 11373129 Review.
-
Intentional maps in posterior parietal cortex.Annu Rev Neurosci. 2002;25:189-220. doi: 10.1146/annurev.neuro.25.112701.142922. Epub 2002 Mar 27. Annu Rev Neurosci. 2002. PMID: 12052908 Review.
Cited by
-
Spatiotemporal trajectories of reactivation of somatosensory cortex by direct and secondary pathways after dorsal column lesions in squirrel monkeys.Neuroimage. 2016 Nov 15;142:431-453. doi: 10.1016/j.neuroimage.2016.08.015. Epub 2016 Aug 12. Neuroimage. 2016. PMID: 27523450 Free PMC article.
-
Neural Basis of Touch and Proprioception in Primate Cortex.Compr Physiol. 2018 Sep 14;8(4):1575-1602. doi: 10.1002/cphy.c170033. Compr Physiol. 2018. PMID: 30215864 Free PMC article. Review.
-
Functional characterization of the fronto-parietal reaching and grasping network: reversible deactivation of M1 and areas 2, 5, and 7b in awake behaving monkeys.J Neurophysiol. 2022 May 1;127(5):1363-1387. doi: 10.1152/jn.00279.2021. Epub 2022 Apr 13. J Neurophysiol. 2022. PMID: 35417261 Free PMC article.
-
The organization and evolution of dorsal stream multisensory motor pathways in primates.Front Neuroanat. 2011 Jun 13;5:34. doi: 10.3389/fnana.2011.00034. eCollection 2011. Front Neuroanat. 2011. PMID: 21716641 Free PMC article.
-
Dynamic reorganization of digit representations in somatosensory cortex of nonhuman primates after spinal cord injury.J Neurosci. 2012 Oct 17;32(42):14649-63. doi: 10.1523/JNEUROSCI.1841-12.2012. J Neurosci. 2012. PMID: 23077051 Free PMC article.
References
-
- Borra E, Belmalih A, Calzavara R, Gerbella M, Murata A, Rozzi S, Luppino G. Cortical connections of the macaque anterior intraparietal (AIP) area. Cereb Cortex. 2008;18:1094–1111. - PubMed
-
- Culham JC, Danckert SL, DeSouza JF, Gati JS, Menon RS, Goodale MA. Visually guided grasping produces fMRI activation in dorsal but not ventral stream brain areas. Exp Brain Res. 2003;153:180–189. - PubMed
-
- Culham JC, Cavina-Pratesi C, Singhal A. The role of parietal cortex in visuomotor control: what have we learned from neuroimaging? Neuropsychologia. 2006;44:2668–2684. - PubMed
-
- Dancause N, Barbay S, Frost SB, Zoubina EV, Plautz EJ, Mahnken JD, Nudo RJ. Effects of small ischemic lesions in the primary motor cortex on neurophysiological organization in ventral premotor cortex. J Neurophysiol. 2006;96:3506–3511. - PubMed
-
- Darian-Smith C. Plasticity of somatosensory function during learning, disease and injury. In: Kaas J, Gardner E, editors. The senses: a comprehensive reference. London: Elsevier; 2008. pp. 259–297.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
