Oxysterol binding protein-dependent activation of sphingomyelin synthesis in the golgi apparatus requires phosphatidylinositol 4-kinase IIα
- PMID: 20881054
- PMCID: PMC2993743
- DOI: 10.1091/mbc.E10-05-0424
Oxysterol binding protein-dependent activation of sphingomyelin synthesis in the golgi apparatus requires phosphatidylinositol 4-kinase IIα
Abstract
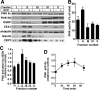
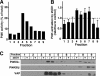
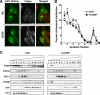
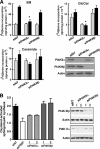
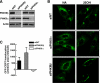
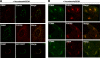
Cholesterol and sphingomyelin (SM) associate in raft domains and are metabolically coregulated. One aspect of coordinate regulation occurs in the Golgi apparatus where oxysterol binding protein (OSBP) mediates sterol-dependent activation of ceramide transport protein (CERT) activity and SM synthesis. Because CERT transfer activity is dependent on its phosphatidylinositol 4 phosphate [PtdIns(4)P]-specific pleckstrin homology domain, we investigated whether OSBP activation of CERT involved a Golgi-associated PtdIns 4-kinase (PI4K). Cell fractionation experiments revealed that Golgi/endosome-enriched membranes from 25-hydroxycholesterol-treated Chinese hamster ovary cells had increased activity of a sterol-sensitive PI4K that was blocked by small interfering RNA silencing of OSBP. Consistent with this sterol-requirement, OSBP silencing also reduced the cholesterol content of endosome/trans-Golgi network (TGN) fractions containing PI4KIIα. PI4KIIα, but not PI4KIIIβ, was required for oxysterol-activation of SM synthesis and recruitment of CERT to the Golgi apparatus. However, neither PI4KIIα nor PI4KIIIβ expression was required for 25-hydroxycholesterol-dependent translocation of OSBP to the Golgi apparatus. The presence of OSBP, CERT, and PI4KIIα in the TGN of oxysterol-stimulated cells suggests that OSBP couples sterol binding or transfer activity with regulation of PI4KIIα activity, leading to CERT recruitment to the TGN and increased SM synthesis.
Figures
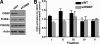
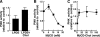
Similar articles
-
Cholesterol regulates oxysterol binding protein (OSBP) phosphorylation and Golgi localization in Chinese hamster ovary cells: correlation with stimulation of sphingomyelin synthesis by 25-hydroxycholesterol.Biochem J. 1998 Nov 15;336 ( Pt 1)(Pt 1):247-56. doi: 10.1042/bj3360247. Biochem J. 1998. PMID: 9806908 Free PMC article.
-
Oxysterol-binding Protein Activation at Endoplasmic Reticulum-Golgi Contact Sites Reorganizes Phosphatidylinositol 4-Phosphate Pools.J Biol Chem. 2016 Jan 15;291(3):1336-47. doi: 10.1074/jbc.M115.682997. Epub 2015 Nov 23. J Biol Chem. 2016. PMID: 26601944 Free PMC article.
-
Oxysterol-binding protein and vesicle-associated membrane protein-associated protein are required for sterol-dependent activation of the ceramide transport protein.Mol Biol Cell. 2006 Jun;17(6):2604-16. doi: 10.1091/mbc.e06-01-0060. Epub 2006 Mar 29. Mol Biol Cell. 2006. PMID: 16571669 Free PMC article.
-
Structure, functions and regulation of CERT, a lipid-transfer protein for the delivery of ceramide at the ER-Golgi membrane contact sites.FEBS Lett. 2019 Sep;593(17):2366-2377. doi: 10.1002/1873-3468.13511. Epub 2019 Jul 8. FEBS Lett. 2019. PMID: 31254361 Review.
-
Phosphoinositides in the hepatitis C virus life cycle.Viruses. 2012 Oct 19;4(10):2340-58. doi: 10.3390/v4102340. Viruses. 2012. PMID: 23202467 Free PMC article. Review.
Cited by
-
The role of phosphatidylinositol 4-kinases and phosphatidylinositol 4-phosphate during viral replication.Biochem Pharmacol. 2012 Dec 1;84(11):1400-8. doi: 10.1016/j.bcp.2012.07.034. Epub 2012 Aug 8. Biochem Pharmacol. 2012. PMID: 22885339 Free PMC article. Review.
-
Insights into the mechanisms of sterol transport between organelles.Cell Mol Life Sci. 2013 Sep;70(18):3405-21. doi: 10.1007/s00018-012-1247-3. Epub 2013 Jan 3. Cell Mol Life Sci. 2013. PMID: 23283302 Free PMC article. Review.
-
Oxysterols and their cellular effectors.Biomolecules. 2012 Feb 15;2(1):76-103. doi: 10.3390/biom2010076. Biomolecules. 2012. PMID: 24970128 Free PMC article.
-
Oxysterol-binding protein family I is the target of minor enviroxime-like compounds.J Virol. 2013 Apr;87(8):4252-60. doi: 10.1128/JVI.03546-12. Epub 2013 Jan 30. J Virol. 2013. PMID: 23365445 Free PMC article.
-
Molecular insights into the membrane-associated phosphatidylinositol 4-kinase IIα.Nat Commun. 2014 Mar 28;5:3552. doi: 10.1038/ncomms4552. Nat Commun. 2014. PMID: 24675427 Free PMC article.
References
-
- Balch W. E., Dunphy W. G., Braell W. A., Rothman J. E. Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell. 1984;39:405–416. - PubMed
-
- Balla A., Tuymetova G., Barshishat M., Geiszt M., Balla T. Characterization of type II phosphatidylinositol 4-kinase isoforms reveals association of the enzymes with endosomal vesicular compartments. J. Biol. Chem. 2002;277:20041–20050. - PubMed
-
- Balla A., Tuymetova G., Tsiomenko A., Varnai P., Balla T. A plasma membrane pool of phosphatidylinositol 4-phosphate is generated by phosphatidylinositol 4-kinase type-III alpha: studies with the PH domains of the oxysterol binding protein and FAPP1. Mol. Biol. Cell. 2005;16:1282–1295. - PMC - PubMed
-
- Barylko B., Gerber S. H., Binns D. D., Grichine N., Khvotchev M., Sudhof T. C., Albanesi J. P. A novel family of phosphatidylinositol 4-kinases conserved from yeast to humans. J. Biol. Chem. 2001;276:7705–7708. - PubMed
-
- Bonifacino J. S., Traub L. M. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003;72:395–447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

