Role of the xenobiotic receptor in inflammatory bowel disease
- PMID: 20878756
- PMCID: PMC3013235
- DOI: 10.1002/ibd.21463
Role of the xenobiotic receptor in inflammatory bowel disease
Abstract
Background: Gene-environment interplay modulates inflammatory bowel diseases (IBD). Dioxin-like compounds can activate the aryl hydrocarbon receptor (AhR) and alter macrophage function as well as T-cell polarization. We hypothesized that attenuation of the AhR signaling pathway will ameliorate colitis in a murine model of IBD.
Methods: Dextran sulfate sodium (DSS) colitis was induced in C57BL/6 AhR null mice (AhR(-/-) ), heterozygous mice (AhR(-/+) ), and their wildtype (WT) littermates. Clinical and morphopathological parameters were used to compare the groups.
Patients: AhR pathway activation was analyzed in biopsy specimens from 25 IBD patients and 15 healthy controls.
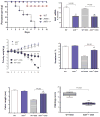
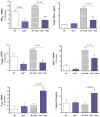
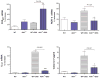
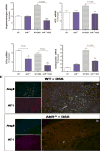
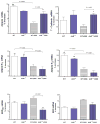
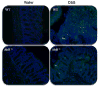
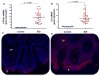
Results: AhR(-/-) mice died before the end of the treatment. However, AhR(-/+) mice exhibited decreased disease activity compared to WT mice. The AhR(-/+) mice expressed less proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α) (6.1- versus 15.7-fold increase) and IL17 (23.7- versus 67.9-fold increase) and increased antiinflammatory IL-10 (2.3-fold increase) compared with the AhR(+/+) mice in the colon. Colonic macrophage infiltration was attenuated in the AhR(-/+) group. AhR and its downstream targets were significantly upregulated in IBD patients versus control (CYP1A1 -19.9, and IL8- 10-fold increase).
Conclusions: Attenuation of the AhR receptor expression resulted in a protective effect during DSS-induced colitis, while the absence of AhR exacerbated the disease. Abnormal AhR pathway activation in the intestinal mucosa of IBD patients may promote chronic inflammation. Modulation of AhR signaling pathway via the diet, cessation of smoking, or administration of AhR antagonists could be viable strategies for the treatment of IBD.
Copyright © 2010 Crohn's & Colitis Foundation of America, Inc.
Figures

Similar articles
-
The aryl hydrocarbon receptor/microRNA-212/132 axis in T cells regulates IL-10 production to maintain intestinal homeostasis.Int Immunol. 2015 Aug;27(8):405-15. doi: 10.1093/intimm/dxv015. Epub 2015 Apr 10. Int Immunol. 2015. PMID: 25862525
-
TolDC Restores the Balance of Th17/Treg via Aryl Hydrocarbon Receptor to Attenuate Colitis.Inflamm Bowel Dis. 2024 Sep 3;30(9):1546-1555. doi: 10.1093/ibd/izae022. Inflamm Bowel Dis. 2024. PMID: 38431309
-
Aryl hydrocarbon receptor confers protection against macrophage pyroptosis and intestinal inflammation through regulating polyamine biosynthesis.Theranostics. 2024 Jul 8;14(11):4218-4239. doi: 10.7150/thno.95749. eCollection 2024. Theranostics. 2024. PMID: 39113799 Free PMC article.
-
Aryl Hydrocarbon Receptor Signalling in the Control of Gut Inflammation.Int J Mol Sci. 2024 Apr 20;25(8):4527. doi: 10.3390/ijms25084527. Int J Mol Sci. 2024. PMID: 38674118 Free PMC article. Review.
-
The Aryl Hydrocarbon Receptor (AHR) as a Potential Target for the Control of Intestinal Inflammation: Insights from an Immune and Bacteria Sensor Receptor.Clin Rev Allergy Immunol. 2020 Dec;59(3):382-390. doi: 10.1007/s12016-020-08789-3. Clin Rev Allergy Immunol. 2020. PMID: 32279195 Review.
Cited by
-
Indole and Tryptophan Metabolism: Endogenous and Dietary Routes to Ah Receptor Activation.Drug Metab Dispos. 2015 Oct;43(10):1522-35. doi: 10.1124/dmd.115.064246. Epub 2015 Jun 3. Drug Metab Dispos. 2015. PMID: 26041783 Free PMC article. Review.
-
The Intestinal Barrier and Current Techniques for the Assessment of Gut Permeability.Cells. 2020 Aug 17;9(8):1909. doi: 10.3390/cells9081909. Cells. 2020. PMID: 32824536 Free PMC article. Review.
-
The Aryl Hydrocarbon Receptor (AhR) Mediates the Counter-Regulatory Effects of Pelargonidins in Models of Inflammation and Metabolic Dysfunctions.Nutrients. 2019 Aug 7;11(8):1820. doi: 10.3390/nu11081820. Nutrients. 2019. PMID: 31394746 Free PMC article.
-
Effects of Milk-Derived Extracellular Vesicles on the Colonic Transcriptome and Proteome in Murine Model.Nutrients. 2022 Jul 26;14(15):3057. doi: 10.3390/nu14153057. Nutrients. 2022. PMID: 35893911 Free PMC article.
-
Interactions between gut microbiota and Parkinson's disease: The role of microbiota-derived amino acid metabolism.Front Aging Neurosci. 2022 Nov 2;14:976316. doi: 10.3389/fnagi.2022.976316. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36408101 Free PMC article. Review.
References
-
- Marquez A, Orozco G, Martinez A, et al. Novel association of the interleukin 2-interleukin 21 region with inflammatory bowel disease. Am J Gastroenterol. 2009;104(8):1968–75. - PubMed
-
- Arsenescu R, Bruno ME, Kaetzel CS, et al. Signature biomarkers in Crohn’s disease: toward a molecular classification. Mucosal Immunol. 2008;1(5):399–411. - PubMed
-
- Bjorksten B. Disease outcomes as a consequence of environmental influences on the development of the immune system. Curr Opin Allergy Clin Immunol. 2009;9(3):185–9. - PubMed
-
- Longstreth GF, Thompson WG, Spiller RC, et al. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
