Effects of eupatilin and jaceosidin on cytochrome p450 enzyme activities in human liver microsomes
- PMID: 20877236
- PMCID: PMC6257796
- DOI: 10.3390/molecules15096466
Effects of eupatilin and jaceosidin on cytochrome p450 enzyme activities in human liver microsomes
Abstract
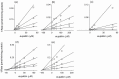
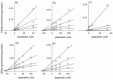
Eupatilin and jaceosidin are bioactive flavones found in the medicinal herbs of the genus Artemisia. These bioactive flavones exhibit various antioxidant, antiinflammatory, antiallergic, and antitumor activities. The inhibitory potentials of eupatilin and jaceosidin on the activities of seven major human cytochrome P450 enzymes in human liver microsomes were investigated using a cocktail probe assay. Eupatilin and jaceosidin potently inhibited CYP1A2-catalyzed phenacetin O-deethylation with 50% inhibitory concentration (IC(50)) values of 9.4 microM and 5.3 microM, respectively, and CYP2C9-catalyzed diclofenac 4-hydroxylation with IC(50) values of 4.1 microM and 10.2 microM, respectively. Eupatilin and jaceosidin were also found to moderately inhibit CYP2C19-catalyzed [S]-mephenytoin 4'-hydroxylation, CYP2D6-catalyzed bufuralol 1'-hydroxylation, and CYP2C8-catalyzed amodiaquine N-deethylation. Kinetic analysis of human liver microsomes showed that eupatilin is a competitive inhibitor of CYP1A2 with a K(i) value of 2.3 microM and a mixed-type inhibitor of CYP2C9 with a K(i) value of 1.6 microM. Jaceosidin was shown to be a competitive inhibitor of CYP1A2 with a K(i) value of 3.8 microM and a mixed-type inhibitor of CYP2C9 with K(i) value of 6.4 microM in human liver microsomes. These in vitro results suggest that eupatilin and jaceosidin should be further examined for potential pharmacokinetic drug interactions in vivo due to inhibition of CYP1A2 and CYP2C9.
Figures
Similar articles
-
Gemfibrozil is a potent inhibitor of human cytochrome P450 2C9.Drug Metab Dispos. 2001 Nov;29(11):1359-61. Drug Metab Dispos. 2001. PMID: 11602509
-
Effect of honokiol on cytochrome P450 and UDP-glucuronosyltransferase enzyme activities in human liver microsomes.Molecules. 2013 Sep 3;18(9):10681-93. doi: 10.3390/molecules180910681. Molecules. 2013. PMID: 24005963 Free PMC article.
-
Interaction of delavirdine with human liver microsomal cytochrome P450: inhibition of CYP2C9, CYP2C19, and CYP2D6.Drug Metab Dispos. 2001 Jan;29(1):41-7. Drug Metab Dispos. 2001. PMID: 11124228
-
Isoniazid is a mechanism-based inhibitor of cytochrome P450 1A2, 2A6, 2C19 and 3A4 isoforms in human liver microsomes.Eur J Clin Pharmacol. 2002 Jan;57(11):799-804. doi: 10.1007/s00228-001-0396-3. Eur J Clin Pharmacol. 2002. PMID: 11868802
-
Inhibitory Effects of Dimethyllirioresinol, Epimagnolin A, Eudesmin, Fargesin, and Magnolin on Cytochrome P450 Enzyme Activities in Human Liver Microsomes.Int J Mol Sci. 2017 May 1;18(5):952. doi: 10.3390/ijms18050952. Int J Mol Sci. 2017. PMID: 28468305 Free PMC article.
Cited by
-
Nrf2-mediated mucoprotective and anti-inflammatory actions of Artemisia extracts led to attenuate stress related mucosal damages.J Clin Biochem Nutr. 2015 Mar;56(2):132-42. doi: 10.3164/jcbn.14-76. Epub 2014 Nov 28. J Clin Biochem Nutr. 2015. PMID: 25759519 Free PMC article.
-
Artemisia asiatica Nakai Attenuates the Expression of Proinflammatory Mediators in Stimulated Macrophages Through Modulation of Nuclear Factor-κB and Mitogen-Activated Protein Kinase Pathways.J Med Food. 2015 Aug;18(8):921-8. doi: 10.1089/jmf.2014.3344. Epub 2015 May 19. J Med Food. 2015. PMID: 26061361 Free PMC article.
-
Effect of a New Prokinetic Agent DA-9701 Formulated with Corydalis Tuber and Pharbitidis Semen on Cytochrome P450 and UDP-Glucuronosyltransferase Enzyme Activities in Human Liver Microsomes.Evid Based Complement Alternat Med. 2012;2012:650718. doi: 10.1155/2012/650718. Epub 2012 Apr 2. Evid Based Complement Alternat Med. 2012. PMID: 22548118 Free PMC article.
-
Complementary medicines used in ulcerative colitis and unintended interactions with cytochrome P450-dependent drug-metabolizing enzymes.Turk J Med Sci. 2022 Oct;52(5):1425-1447. doi: 10.55730/1300-0144.5482. Epub 2022 Oct 19. Turk J Med Sci. 2022. PMID: 36422483 Free PMC article. Review.
-
Corydaline inhibits multiple cytochrome P450 and UDP-glucuronosyltransferase enzyme activities in human liver microsomes.Molecules. 2011 Aug 5;16(8):6591-602. doi: 10.3390/molecules16086591. Molecules. 2011. PMID: 21826053 Free PMC article.
References
-
- Kim A.R., Zou Y.N., Park T.H., Shim K.H., Kim M.S., Kim N.D., Kim J.D., Bae S.J., Choi J.S., Chung H.Y. Active components from Artemisia iwayomogi displaying ONOO(-) scavenging activity. Phytother. Res. 2004;18:1–7. - PubMed
-
- Moscatelli V., Hnatyszyn O., Acevedo C., Megias J., Alcaraz M.J., Ferraro G. Flavonoids from Artemisia copa with anti-inflammatory activity. Planta Med. 2006;72:72–74. - PubMed
-
- Choi E.J., Oh H.M., Na B.R., Ramesh T.P., Lee H.J., Choi C.S., Choi S.C., Kim K.H., Oh T.Y., Choi S.J., Chae J.R., Kim S.W., Jun C.D. Eupatilin protects gastric epithelial cells from oxidative damage and down-regulates genes responsible for the cellular oxidative stress. Pharm. Res. 2008;25:1355–1364. doi: 10.1007/s11095-008-9531-5. - DOI - PubMed
-
- Kim M.J., Han J.M., Jin Y.Y., Baek N.I., Bang M.H., Chung H.G., Choi M.S., Lee K.T., Sok D.E., Jeong T.S. In vitro antioxidant and anti-inflammatory activities of jaceosidin from Artemisia princeps Pampanini cv. Sajabal. Arch. Pharm. Res. 2008;31:429–437. doi: 10.1007/s12272-001-1175-8. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

