Abba promotes PDGF-mediated membrane ruffling through activation of the small GTPase Rac1
- PMID: 20875796
- PMCID: PMC2980902
- DOI: 10.1016/j.bbrc.2010.09.087
Abba promotes PDGF-mediated membrane ruffling through activation of the small GTPase Rac1
Abstract
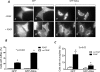
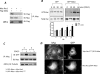
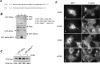
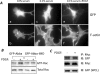
Abba is a member of the I-BAR-domain protein family that is characterized by a convex-shaped membrane-binding motif. Overexpression of GFP-tagged Abba in murine fibroblasts potentiated PDGF-mediated formation of membrane ruffles and lamellipodia. Immunofluorescent microscopy and pull-down analysis revealed that GFP-Abba colocalized with an active form of Rac1 in the membrane ruffles and enhanced the Rac GTPase activity in response to PDGF stimulation. Further immunoprecipitation assays demonstrated that GFP-Abba bound to both wild-type and constitutively active Rac1 and that the binding to either of the Rac1 forms was significantly enhanced upon PDGF stimulation. On the other hand, an Abba mutant deficient in Rac1 binding failed to promote membrane ruffling and Rac1 activation in response to PDGF. However, the cells overexpressing a truncated mutant carrying the I-BAR domain alone displayed numerous filopodia-like microspikes in a manner independent of growth factors. Also, the Rac-binding activity of the mutant was not affected by PDGF treatment. Our data indicates that the interaction between full-length Abba and Rac1 is implicated in membrane deformation and subjected to a growth factor-mediated regulation through the C-terminal sequence.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Fibronectin-mediated cell spreading requires ABBA-Rac1 signaling.J Cell Biochem. 2013 Apr;114(4):773-81. doi: 10.1002/jcb.24415. J Cell Biochem. 2013. PMID: 23060091
-
Translocation of cortactin to the cell periphery is mediated by the small GTPase Rac1.J Cell Sci. 1998 Aug;111 ( Pt 16):2433-43. doi: 10.1242/jcs.111.16.2433. J Cell Sci. 1998. PMID: 9683637
-
Mechanisms of guanine nucleotide exchange and Rac-mediated signaling revealed by a dominant negative trio mutant.J Biol Chem. 2004 Jan 30;279(5):3777-86. doi: 10.1074/jbc.M308282200. Epub 2003 Nov 3. J Biol Chem. 2004. PMID: 14597635
-
RhoG GTPase controls a pathway that independently activates Rac1 and Cdc42Hs.Mol Biol Cell. 1998 Jun;9(6):1379-94. doi: 10.1091/mbc.9.6.1379. Mol Biol Cell. 1998. PMID: 9614181 Free PMC article.
-
Phosphatidylinositol 3-kinase-dependent membrane recruitment of Rac-1 and p47phox is critical for alpha-platelet-derived growth factor receptor-induced production of reactive oxygen species.J Biol Chem. 2008 Mar 21;283(12):7864-76. doi: 10.1074/jbc.M704997200. Epub 2007 Dec 10. J Biol Chem. 2008. PMID: 18070887
Cited by
-
Differential interactions of missing in metastasis and insulin receptor tyrosine kinase substrate with RAB proteins in the endocytosis of CXCR4.J Biol Chem. 2019 Apr 19;294(16):6494-6505. doi: 10.1074/jbc.RA118.006071. Epub 2019 Feb 26. J Biol Chem. 2019. PMID: 30808710 Free PMC article.
-
Exosomal microRNA-92b Is a Diagnostic Biomarker in Breast Cancer and Targets Survival-Related MTSS1L to Promote Tumorigenesis.Int J Mol Sci. 2024 Jan 20;25(2):1295. doi: 10.3390/ijms25021295. Int J Mol Sci. 2024. PMID: 38279296 Free PMC article.
-
Interaction of crk with Myosin-1c participates in fibronectin-induced cell spreading.Int J Biol Sci. 2013 Aug 15;9(8):778-91. doi: 10.7150/ijbs.6459. eCollection 2013. Int J Biol Sci. 2013. PMID: 23983611 Free PMC article.
-
Control of Rho GTPase function by BAR-domains.Small GTPases. 2012 Jan-Mar;3(1):45-52. doi: 10.4161/sgtp.18960. Small GTPases. 2012. PMID: 22714417 Free PMC article.
-
BAR domain proteins-a linkage between cellular membranes, signaling pathways, and the actin cytoskeleton.Biophys Rev. 2018 Dec;10(6):1587-1604. doi: 10.1007/s12551-018-0467-7. Epub 2018 Nov 19. Biophys Rev. 2018. PMID: 30456600 Free PMC article. Review.
References
-
- Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303:495–499. - PubMed
-
- Gallop JL, McMahon HT. BAR domains and membrane curvature: bringing your curves to the BAR. Biochem.Soc.Symp. 2005:223–231. - PubMed
-
- Saarikangas J, Zhao H, Pykalainen A, Laurinmaki P, Mattila PK, Kinnunen PK, Butcher SJ, Lappalainen P. Molecular mechanisms of membrane deformation by I-BAR domain proteins. Curr.Biol. 2009;19:95–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

