The role of the c-Jun N-terminal kinase 2-α-isoform in non-small cell lung carcinoma tumorigenesis
- PMID: 20871632
- PMCID: PMC5661974
- DOI: 10.1038/onc.2010.414
The role of the c-Jun N-terminal kinase 2-α-isoform in non-small cell lung carcinoma tumorigenesis
Abstract
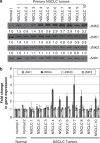
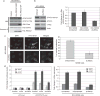
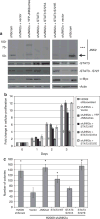
The c-Jun N-terminal kinases (JNKs) are members of the mitogen-activated protein kinase family and have been implicated in tumorigenesis. One isoform in particular, JNK2α, has been shown to be frequently activated in primary brain tumors, to enhance several tumorigenic phenotypes and to increase tumor formation in mice. As JNK is frequently activated in non-small cell lung carcinoma (NSCLC), we investigated the role of the JNK2α isoform in NSCLC formation by examining its expression in primary tumors and by modulating its expression in cultured cell lines. We discovered that 60% of the tested primary NSCLC tumors had three-fold higher JNK2 protein and two- to three-fold higher JNK2α mRNA expression than normal lung control tissue. To determine the importance of JNK2α in NSCLC progression, we reduced JNK2α expression in multiple NSCLC cell lines using short hairpin RNA. Cell lines deficient in JNK2α had decreased cellular growth and anchorage-independent growth, and the tumors were four-fold smaller in mass. To elucidate the mechanism by which JNK2α induces NSCLC growth, we analyzed the JNK substrate, signal transducer and activator of transcription 3 (STAT3). Our data demonstrates for the first time that JNK2α can regulate the transcriptional activity of STAT3 by phosphorylating the Ser727 residue, thereby regulating the expression of oncogenic genes, such as c-Myc. Furthermore, reintroduction of JNK2α2 or STAT3 restored the tumorigenicity of the NSCLC cells, demonstrating that JNK2α is important for NSCLC progression. Our studies reveal a novel mechanism in which phosphorylation of STAT3 is mediated by a constitutively active JNK2 isoform, JNK2α.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


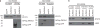
Similar articles
-
c-Jun NH(2)-terminal kinase 2alpha2 promotes the tumorigenicity of human glioblastoma cells.Cancer Res. 2006 Oct 15;66(20):10024-31. doi: 10.1158/0008-5472.CAN-06-0136. Cancer Res. 2006. PMID: 17047065
-
c-Jun N-terminal kinase is activated in non-small-cell lung cancer and promotes neoplastic transformation in human bronchial epithelial cells.Oncogene. 2007 Apr 19;26(18):2658-66. doi: 10.1038/sj.onc.1210050. Epub 2006 Oct 23. Oncogene. 2007. PMID: 17057737
-
Small-molecule targeting of signal transducer and activator of transcription (STAT) 3 to treat non-small cell lung cancer.Lung Cancer. 2015 Nov;90(2):182-90. doi: 10.1016/j.lungcan.2015.09.014. Epub 2015 Sep 15. Lung Cancer. 2015. PMID: 26410177 Free PMC article.
-
Signal transducer and activator of transcription 3 as molecular therapy for non-small-cell lung cancer.J Thorac Oncol. 2014 Apr;9(4):488-96. doi: 10.1097/JTO.0000000000000107. J Thorac Oncol. 2014. PMID: 24736071
-
JNK signaling mediates EPHA2-dependent tumor cell proliferation, motility, and cancer stem cell-like properties in non-small cell lung cancer.Cancer Res. 2014 May 1;74(9):2444-54. doi: 10.1158/0008-5472.CAN-13-2136. Epub 2014 Mar 7. Cancer Res. 2014. PMID: 24607842 Free PMC article.
Cited by
-
JNK signalling in cancer: in need of new, smarter therapeutic targets.Br J Pharmacol. 2014 Jan;171(1):24-37. doi: 10.1111/bph.12432. Br J Pharmacol. 2014. PMID: 24117156 Free PMC article. Review.
-
SIFORM: shared informative factor models for integration of multi-platform bioinformatic data.Bioinformatics. 2016 Nov 1;32(21):3279-3290. doi: 10.1093/bioinformatics/btw295. Epub 2016 Jul 5. Bioinformatics. 2016. PMID: 27381342 Free PMC article.
-
γ-Glutamyl transferase 7 is a novel regulator of glioblastoma growth.BMC Cancer. 2015 Apr 7;15:225. doi: 10.1186/s12885-015-1232-y. BMC Cancer. 2015. PMID: 25884624 Free PMC article.
-
Alveolar hypoxia promotes murine lung tumor growth through a VEGFR-2/EGFR-dependent mechanism.Cancer Prev Res (Phila). 2012 Aug;5(8):1061-71. doi: 10.1158/1940-6207.CAPR-12-0069-T. Epub 2012 Jun 14. Cancer Prev Res (Phila). 2012. PMID: 22700853 Free PMC article.
-
A Novel Autophagy-Related Prognostic Risk Model and a Nomogram for Survival Prediction of Oral Cancer Patients.Biomed Res Int. 2022 Jan 6;2022:2067540. doi: 10.1155/2022/2067540. eCollection 2022. Biomed Res Int. 2022. PMID: 35036428 Free PMC article.
References
-
- Achcar Rde O, Cagle PT, Jagirdar J. Expression of activated and latent signal transducer and activator of transcription 3 in 303 non-small cell lung carcinomas and 44 malignant mesotheliomas: possible role for chemotherapeutic intervention. Arch Pathol Lab Med. 2007;131:1350–1360. - PubMed
-
- Alvarez JV, Greulich H, Sellers WR, Meyerson M, Frank DA. Signal transducer and activator of transcription 3 is required for the oncogenic effects of non-small-cell lung cancer-associated mutations of the epidermal growth factor receptor. Cancer Res. 2006;66:3162–3168. - PubMed
-
- Berner JM, Sorlie T, Mertens F, Henriksen J, Saeter G, Mandahl N, et al. Chromosome band 9p21 is frequently altered in malignant peripheral nerve sheath tumors: studies of CDKN2A and other genes of the pRB pathway. Genes Chromosomes Cancer. 1999;26:151–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

