Receptor activator of NF-{kappa}B (RANK) cytoplasmic IVVY535-538 motif plays an essential role in tumor necrosis factor-{alpha} (TNF)-mediated osteoclastogenesis
- PMID: 20870724
- PMCID: PMC2988348
- DOI: 10.1074/jbc.M110.149484
Receptor activator of NF-{kappa}B (RANK) cytoplasmic IVVY535-538 motif plays an essential role in tumor necrosis factor-{alpha} (TNF)-mediated osteoclastogenesis
Abstract
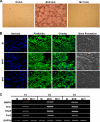
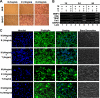
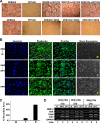
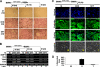
Tumor necrosis factor-α (TNF) enhances osteoclast formation and activity leading to bone loss in various pathological conditions, but its precise role in osteoclastogenesis remains controversial. Although several groups showed that TNF can promote osteoclastogenesis independently of the receptor activator of NF-κB (RANK) ligand (RANKL), others demonstrated that TNF-mediated osteoclastogenesis needs permissive levels of RANKL. Here, we independently reveal that although TNF cannot stimulate osteoclastogenesis on bone slices, it can induce the formation of functional osteoclasts on bone slices in the presence of permissive levels of RANKL or from bone marrow macrophages (BMMs) pretreated by RANKL. TNF can still promote the formation of functional osteoclasts 2 days after transient RANKL pretreatment. These data have confirmed that TNF-mediated osteoclastogenesis requires priming of BMMs by RANKL. Moreover, we investigated the molecular mechanism underlying the dependence of TNF-mediated osteoclastogenesis on RANKL. RANK, the receptor for RANKL, contains an IVVY(535-538) motif that has been shown to play a vital role in osteoclastogenesis by committing BMMs to the osteoclast lineage. We show that TNF-induced osteoclastogenesis depends on RANKL to commit BMMs to the osteoclast lineage and RANKL regulates the lineage commitment through the IVVY motif. Mechanistically, the IVVY motif controls the lineage commitment by reprogramming osteoclast genes into an inducible state in which they can be activated by TNF. Our findings not only provide important mechanistic insights into the action of RANKL in TNF-mediated osteoclastogenesis but also establish that the IVVY motif may serve as an attractive therapeutic target for bone loss in various bone disorders.
Figures



Similar articles
-
Molecular basis of requirement of receptor activator of nuclear factor κB signaling for interleukin 1-mediated osteoclastogenesis.J Biol Chem. 2012 May 4;287(19):15728-38. doi: 10.1074/jbc.M111.296228. Epub 2012 Mar 13. J Biol Chem. 2012. PMID: 22416138 Free PMC article.
-
The IVVY Motif and Tumor Necrosis Factor Receptor-associated Factor (TRAF) Sites in the Cytoplasmic Domain of the Receptor Activator of Nuclear Factor κB (RANK) Cooperate to Induce Osteoclastogenesis.J Biol Chem. 2015 Sep 25;290(39):23738-50. doi: 10.1074/jbc.M115.667535. Epub 2015 Aug 14. J Biol Chem. 2015. PMID: 26276390 Free PMC article.
-
RANK IVVY motif plays crucial roles in osteoclastogenesis.Bone. 2025 Mar;192:117367. doi: 10.1016/j.bone.2024.117367. Epub 2024 Dec 10. Bone. 2025. PMID: 39667419
-
A new member of tumor necrosis factor ligand family, ODF/OPGL/TRANCE/RANKL, regulates osteoclast differentiation and function.Biochem Biophys Res Commun. 1999 Mar 24;256(3):449-55. doi: 10.1006/bbrc.1999.0252. Biochem Biophys Res Commun. 1999. PMID: 10080918 Review.
-
Immunological reaction in TNF-α-mediated osteoclast formation and bone resorption in vitro and in vivo.Clin Dev Immunol. 2013;2013:181849. doi: 10.1155/2013/181849. Epub 2013 May 23. Clin Dev Immunol. 2013. PMID: 23762085 Free PMC article. Review.
Cited by
-
Notch signaling promotes osteoclast maturation and resorptive activity.J Cell Biochem. 2015 Nov;116(11):2598-609. doi: 10.1002/jcb.25205. J Cell Biochem. 2015. PMID: 25914241 Free PMC article.
-
Interaction of Tumor Necrosis Factor Receptor-associated Factor 6 (TRAF6) and Vav3 in the Receptor Activator of Nuclear Factor κB (RANK) Signaling Complex Enhances Osteoclastogenesis.J Biol Chem. 2016 Sep 23;291(39):20643-60. doi: 10.1074/jbc.M116.728303. Epub 2016 Aug 9. J Biol Chem. 2016. PMID: 27507811 Free PMC article.
-
CSTB Downregulation Promotes Cell Proliferation and Migration and Suppresses Apoptosis in Gastric Cancer SGC-7901 Cell Line.Oncol Res. 2016 Oct 27;24(6):487-494. doi: 10.3727/096504016X14685034103752. Oncol Res. 2016. PMID: 28281969 Free PMC article.
-
C/EBPα transcription factor is regulated by the RANK cytoplasmic 535IVVY538 motif and stimulates osteoclastogenesis more strongly than c-Fos.J Biol Chem. 2018 Jan 26;293(4):1480-1492. doi: 10.1074/jbc.M116.736009. Epub 2017 Nov 9. J Biol Chem. 2018. PMID: 29122885 Free PMC article.
-
Genetic ablation of CD68 results in mice with increased bone and dysfunctional osteoclasts.PLoS One. 2011;6(10):e25838. doi: 10.1371/journal.pone.0025838. Epub 2011 Oct 3. PLoS One. 2011. PMID: 21991369 Free PMC article.
References
-
- Beyaert R., Fiers W. (1998) in Cytokines (Mire-Slus A., Thorpe R. eds) pp. 335–360, Academic Press, Orlando, FL
-
- Vassalli P. (1992) Annu. Rev. Immunol. 10, 411–452 - PubMed
-
- Jilka R. L. (1998) Bone 23, 75–81 - PubMed
-
- Goldring S. R. (2003) Rheumatology 42, (Suppl. 2) ii11–ii16 - PubMed
-
- Graves D. T., Cochran D. (2003) J. Periodontol. 74, 391–401 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

