Role of ubiquitination in endocytic trafficking of G-protein-coupled receptors
- PMID: 20854416
- PMCID: PMC3625703
- DOI: 10.1111/j.1600-0854.2010.01121.x
Role of ubiquitination in endocytic trafficking of G-protein-coupled receptors
Abstract
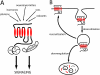
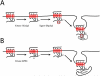
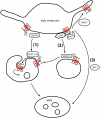
Lysyl ubiquitination has long been known to target cytoplasmic proteins for proteasomal degradation, and there is now extensive evidence that ubiquitination functions in vacuolar/lysosomal targeting of membrane proteins from both the biosynthetic and endocytic pathways. G-protein-coupled receptors (GPCRs) represent the largest and most diverse family of membrane proteins, whose function is of fundamental importance both physiologically and therapeutically. In this review, we discuss the role of ubiquitination in the vacuolar/lysosomal downregulation of GPCRs through the endocytic pathway, with a primary focus on lysosomal trafficking in mammalian cells. We will summarize evidence indicating that mammalian GPCRs are regulated by ubiquitin-dependent mechanisms conserved in budding yeast, and then consider evidence for additional ubiquitin-dependent and -independent regulation that may be specific to animal cells.
© 2010 John Wiley & Sons A/S.
Figures
Similar articles
-
Ubiquitination of G protein-coupled receptors: functional implications and drug discovery.Mol Pharmacol. 2012 Oct;82(4):563-70. doi: 10.1124/mol.112.079418. Epub 2012 Jun 14. Mol Pharmacol. 2012. PMID: 22700696 Free PMC article. Review.
-
Regulation of GPCRs by endocytic membrane trafficking and its potential implications.Annu Rev Pharmacol Toxicol. 2008;48:537-68. doi: 10.1146/annurev.pharmtox.48.113006.094830. Annu Rev Pharmacol Toxicol. 2008. PMID: 18184106 Review.
-
Role of mammalian vacuolar protein-sorting proteins in endocytic trafficking of a non-ubiquitinated G protein-coupled receptor to lysosomes.J Biol Chem. 2004 May 21;279(21):22522-31. doi: 10.1074/jbc.M311062200. Epub 2004 Mar 15. J Biol Chem. 2004. PMID: 15024011
-
Minireview: ubiquitination-regulated G protein-coupled receptor signaling and trafficking.Mol Endocrinol. 2013 Apr;27(4):558-72. doi: 10.1210/me.2012-1404. Epub 2013 Mar 7. Mol Endocrinol. 2013. PMID: 23471539 Free PMC article. Review.
-
Ubiquitin-dependent regulation of G protein-coupled receptor trafficking and signaling.Cell Signal. 2013 Mar;25(3):707-16. doi: 10.1016/j.cellsig.2012.11.024. Epub 2012 Nov 29. Cell Signal. 2013. PMID: 23201781 Free PMC article. Review.
Cited by
-
Ubiquitin-mediated regulation of endocytosis by proteins of the arrestin family.Biochem Res Int. 2012;2012:242764. doi: 10.1155/2012/242764. Epub 2012 Sep 4. Biochem Res Int. 2012. PMID: 22988512 Free PMC article.
-
Regulation of endocytic clathrin dynamics by cargo ubiquitination.Dev Cell. 2012 Sep 11;23(3):519-32. doi: 10.1016/j.devcel.2012.08.003. Epub 2012 Aug 30. Dev Cell. 2012. PMID: 22940114 Free PMC article.
-
Epigenetic meta-analysis across three civilian cohorts identifies NRG1 and HGS as blood-based biomarkers for post-traumatic stress disorder.Epigenomics. 2018 Dec;10(12):1585-1601. doi: 10.2217/epi-2018-0049. Epub 2018 Nov 20. Epigenomics. 2018. PMID: 30456986 Free PMC article.
-
The intralumenal fragment pathway mediates ESCRT-independent surface transporter down-regulation.Nat Commun. 2018 Dec 18;9(1):5358. doi: 10.1038/s41467-018-07734-5. Nat Commun. 2018. PMID: 30560896 Free PMC article.
-
Mammalian α arrestins link activated seven transmembrane receptors to Nedd4 family e3 ubiquitin ligases and interact with β arrestins.PLoS One. 2012;7(12):e50557. doi: 10.1371/journal.pone.0050557. Epub 2012 Dec 7. PLoS One. 2012. PMID: 23236378 Free PMC article.
References
-
- Jacoby E, Bouhelal R, Gerspacher M, Seuwen K. The 7 TM G-protein-coupled receptor target family. ChemMedChem. 2006;1(8):761–782. - PubMed
-
- Fredholm BB, Hokfelt T, Milligan G. G-protein-coupled receptors: an update. Acta Physiol (Oxf) 2007;190(1):3–7. - PubMed
-
- Brzostowski JA, Kimmel AR. Signaling at zero G: G-protein-independent functions for 7-TM receptors. Trends Biochem Sci. 2001;26(5):291–297. - PubMed
-
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

