IL-21 deficiency influences CD8 T cell quality and recall responses following an acute viral infection
- PMID: 20844201
- PMCID: PMC2950881
- DOI: 10.4049/jimmunol.1001032
IL-21 deficiency influences CD8 T cell quality and recall responses following an acute viral infection
Abstract
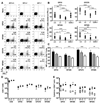
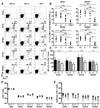
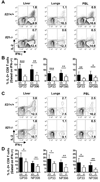
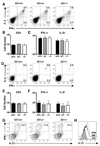
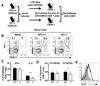
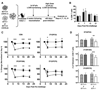
CD4 T cells are principal producers of IL-21 and are often required for optimal CD8 T cell responses. Therefore, we investigated the importance of IL-21 in determining the phenotypic attributes, functional quality, and maintenance of antiviral CD8 T cells following acute infection with the prototypic mouse pathogen lymphocytic choriomeningitis virus. Previous reports have documented an obligatory role for IL-21 in sustaining CD8 T cell responses during chronic infections. Here we show that the requirements for IL-21 are less stringent following acute infections; however, in the absence of IL-21, the capacity of CD8 T cells to attain the polyfunctional trait of IL-2 production is consistently reduced during both the effector and memory phases. This is further supported by in vitro studies showing that the addition of IL-21 promotes the differentiation of IL-2-producing CD8 T cells. Although the generation of memory CD8 T cells, which are capable of mounting protective recall responses, proceeds independently of IL-21, we demonstrate that IL-21 does function to support secondary responses, especially under competitive conditions. Collectively, these studies highlight the potential roles of IL-21 in determining the quality of CD8 T cell responses postinfection.
Figures




Similar articles
-
Antiviral CD4 and CD8 T-cell memory: differences in the size of the response and activation requirements.Philos Trans R Soc Lond B Biol Sci. 2000 Mar 29;355(1395):373-9. doi: 10.1098/rstb.2000.0577. Philos Trans R Soc Lond B Biol Sci. 2000. PMID: 10794058 Free PMC article.
-
A vital role for interleukin-21 in the control of a chronic viral infection.Science. 2009 Jun 19;324(5934):1572-6. doi: 10.1126/science.1175194. Epub 2009 May 14. Science. 2009. PMID: 19443735 Free PMC article.
-
IL-10 Regulates Memory T Cell Development and the Balance between Th1 and Follicular Th Cell Responses during an Acute Viral Infection.J Immunol. 2016 Aug 15;197(4):1308-21. doi: 10.4049/jimmunol.1502481. Epub 2016 Jul 8. J Immunol. 2016. PMID: 27402701 Free PMC article.
-
A Context-Dependent Role for IL-21 in Modulating the Differentiation, Distribution, and Abundance of Effector and Memory CD8 T Cell Subsets.J Immunol. 2016 Mar 1;196(5):2153-66. doi: 10.4049/jimmunol.1401236. Epub 2016 Jan 29. J Immunol. 2016. PMID: 26826252 Free PMC article.
-
Interleukin-21-dependent modulation of T cell antigen receptor reactivity towards low affinity peptide ligands in autoreactive CD8(+) T lymphocytes.Cytokine. 2016 Sep;85:83-91. doi: 10.1016/j.cyto.2016.06.011. Epub 2016 Jun 11. Cytokine. 2016. PMID: 27300756
Cited by
-
Tailored immune responses: novel effector helper T cell subsets in protective immunity.PLoS Pathog. 2014 Feb 20;10(2):e1003905. doi: 10.1371/journal.ppat.1003905. eCollection 2014 Feb. PLoS Pathog. 2014. PMID: 24586147 Free PMC article. Review.
-
Interleukin-21 combined with ART reduces inflammation and viral reservoir in SIV-infected macaques.J Clin Invest. 2015 Dec;125(12):4497-513. doi: 10.1172/JCI81400. Epub 2015 Nov 9. J Clin Invest. 2015. PMID: 26551680 Free PMC article.
-
IL-21R expression on CD8+ T cells promotes CD8+ T cell activation in coxsackievirus B3 induced myocarditis.Exp Mol Pathol. 2012 Jun;92(3):327-33. doi: 10.1016/j.yexmp.2012.03.009. Epub 2012 Mar 21. Exp Mol Pathol. 2012. PMID: 22465422 Free PMC article.
-
Interleukin-21 and T follicular helper cells in HIV infection: research focus and future perspectives.Immunol Res. 2013 Dec;57(1-3):279-91. doi: 10.1007/s12026-013-8457-0. Immunol Res. 2013. PMID: 24242760 Free PMC article. Review.
-
Choline acetyltransferase-expressing T cells are required to control chronic viral infection.Science. 2019 Feb 8;363(6427):639-644. doi: 10.1126/science.aau9072. Science. 2019. PMID: 30733420 Free PMC article.
References
-
- Lau LL, Jamieson BD, Somasundaram T, Ahmed R. Cytotoxic T-cell memory without antigen. Nature. 1994;369:648–652. - PubMed
-
- Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. - PubMed
-
- Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med. 2001;7:913–919. - PubMed
-
- Kristensen NN, Christensen JP, Thomsen AR. High numbers of IL-2-producing CD8+ T cells during viral infection: correlation with stable memory development. J Gen Virol. 2002;83:2123–2133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

