Role of alpha7 nicotinic acetylcholine receptor in calcium signaling induced by prion protein interaction with stress-inducible protein 1
- PMID: 20837487
- PMCID: PMC2978582
- DOI: 10.1074/jbc.M110.157263
Role of alpha7 nicotinic acetylcholine receptor in calcium signaling induced by prion protein interaction with stress-inducible protein 1
Abstract
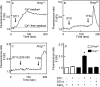
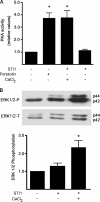
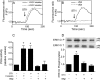
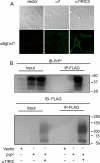
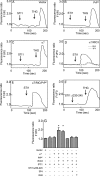
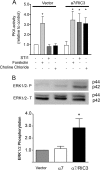
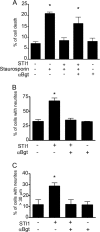
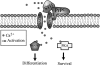
The prion protein (PrP(C)) is a conserved glycosylphosphatidylinositol-anchored cell surface protein expressed by neurons and other cells. Stress-inducible protein 1 (STI1) binds PrP(C) extracellularly, and this activated signaling complex promotes neuronal differentiation and neuroprotection via the extracellular signal-regulated kinase 1 and 2 (ERK1/2) and cAMP-dependent protein kinase 1 (PKA) pathways. However, the mechanism by which the PrP(C)-STI1 interaction transduces extracellular signals to the intracellular environment is unknown. We found that in hippocampal neurons, STI1-PrP(C) engagement induces an increase in intracellular Ca(2+) levels. This effect was not detected in PrP(C)-null neurons or wild-type neurons treated with an STI1 mutant unable to bind PrP(C). Using a best candidate approach to test for potential channels involved in Ca(2+) influx evoked by STI1-PrP(C), we found that α-bungarotoxin, a specific inhibitor for α7 nicotinic acetylcholine receptor (α7nAChR), was able to block PrP(C)-STI1-mediated signaling, neuroprotection, and neuritogenesis. Importantly, when α7nAChR was transfected into HEK 293 cells, it formed a functional complex with PrP(C) and allowed reconstitution of signaling by PrP(C)-STI1 interaction. These results indicate that STI1 can interact with the PrP(C)·α7nAChR complex to promote signaling and provide a novel potential target for modulation of the effects of prion protein in neurodegenerative diseases.
Figures
Similar articles
-
Laminin-γ1 chain and stress inducible protein 1 synergistically mediate PrPC-dependent axonal growth via Ca2+ mobilization in dorsal root ganglia neurons.J Neurochem. 2013 Jan;124(2):210-23. doi: 10.1111/jnc.12091. Epub 2012 Dec 4. J Neurochem. 2013. PMID: 23145988
-
Endocytosis of prion protein is required for ERK1/2 signaling induced by stress-inducible protein 1.J Neurosci. 2008 Jun 25;28(26):6691-702. doi: 10.1523/JNEUROSCI.1701-08.2008. J Neurosci. 2008. PMID: 18579743 Free PMC article.
-
Loss of STI1-mediated neuronal survival and differentiation in disease-associated mutations of prion protein.J Neurochem. 2018 Jun;145(5):409-416. doi: 10.1111/jnc.14305. Epub 2018 Apr 2. J Neurochem. 2018. PMID: 29337365
-
Cellular prion protein signaling in serotonergic neuronal cells.Ann N Y Acad Sci. 2007 Jan;1096:106-19. doi: 10.1196/annals.1397.076. Ann N Y Acad Sci. 2007. PMID: 17405922 Review.
-
Dual role of cellular prion protein in normal host and Alzheimer's disease.Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(4):155-173. doi: 10.2183/pjab.93.010. Proc Jpn Acad Ser B Phys Biol Sci. 2017. PMID: 28413194 Free PMC article. Review.
Cited by
-
Uncontrolled SFK-mediated protein trafficking in prion and Alzheimer's disease.Prion. 2016 Sep 2;10(5):352-361. doi: 10.1080/19336896.2016.1221873. Prion. 2016. PMID: 27649856 Free PMC article.
-
Double-Edge Sword of Sustained ROCK Activation in Prion Diseases through Neuritogenesis Defects and Prion Accumulation.PLoS Pathog. 2015 Aug 4;11(8):e1005073. doi: 10.1371/journal.ppat.1005073. eCollection 2015 Aug. PLoS Pathog. 2015. PMID: 26241960 Free PMC article.
-
The Quest for Cellular Prion Protein Functions in the Aged and Neurodegenerating Brain.Cells. 2020 Mar 2;9(3):591. doi: 10.3390/cells9030591. Cells. 2020. PMID: 32131451 Free PMC article. Review.
-
Stress-inducible phosphoprotein 1 (HOP/STI1/STIP1) regulates the accumulation and toxicity of α-synuclein in vivo.Acta Neuropathol. 2022 Nov;144(5):881-910. doi: 10.1007/s00401-022-02491-8. Epub 2022 Sep 19. Acta Neuropathol. 2022. PMID: 36121476 Free PMC article.
-
Hijacking PrP(c)-dependent signal transduction: when prions impair Aβ clearance.Front Aging Neurosci. 2014 Feb 28;6:25. doi: 10.3389/fnagi.2014.00025. eCollection 2014. Front Aging Neurosci. 2014. PMID: 24592237 Free PMC article. Review.
References
-
- Linden R., Martins V. R., Prado M. A., Cammarota M., Izquierdo I., Brentani R. R. (2008) Physiol. Rev. 88, 673–728 - PubMed
-
- Linden R., Martins V. R, Prado M. A. (2009) Prion protein. UCSD-Nature Molecule Pages, 29July2009, doi:10.1038/mp.a003935.01 - DOI
-
- Nimmrich V., Ebert U. (2009) Rev. Neurosci. 20, 1–12 - PubMed
-
- Walsh D. M., Selkoe D. J. (2007) J. Neurochem. 101, 1172–1184 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

