Heterocellular induction of interferon by negative-sense RNA viruses
- PMID: 20833406
- PMCID: PMC2963793
- DOI: 10.1016/j.virol.2010.08.008
Heterocellular induction of interferon by negative-sense RNA viruses
Abstract
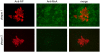
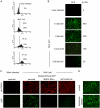
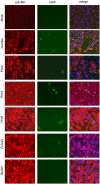
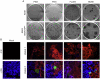
The infection of cells by RNA viruses is associated with the recognition of virus PAMPs (pathogen-associated molecular patterns) and the production of type I interferon (IFN). To counter this, most, if not all, RNA viruses encode antagonists of the IFN system. Here we present data on the dynamics of IFN production and response during developing infections by paramyxoviruses, influenza A virus and bunyamwera virus. We show that only a limited number of infected cells are responsible for the production of IFN, and that this heterocellular production is a feature of the infecting virus as opposed to an intrinsic property of the cells.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Bunyamwera bunyavirus nonstructural protein NSs counteracts the induction of alpha/beta interferon.J Virol. 2002 Aug;76(16):7949-55. doi: 10.1128/jvi.76.16.7949-7955.2002. J Virol. 2002. PMID: 12133999 Free PMC article.
-
The regulation of type I interferon production by paramyxoviruses.J Interferon Cytokine Res. 2009 Sep;29(9):539-47. doi: 10.1089/jir.2009.0071. J Interferon Cytokine Res. 2009. PMID: 19702509 Free PMC article. Review.
-
NS1 proteins of avian influenza A viruses can act as antagonists of the human alpha/beta interferon response.J Virol. 2007 Mar;81(5):2318-27. doi: 10.1128/JVI.01856-06. Epub 2006 Dec 20. J Virol. 2007. PMID: 17182679 Free PMC article.
-
Differences in Type I interferon response in human lung epithelial cells infected by highly pathogenic H5N1 and low pathogenic H11N1 avian influenza viruses.Virus Genes. 2018 Jun;54(3):414-423. doi: 10.1007/s11262-018-1556-1. Epub 2018 Mar 24. Virus Genes. 2018. PMID: 29574656
-
Paramyxovirus activation and inhibition of innate immune responses.J Mol Biol. 2013 Dec 13;425(24):4872-92. doi: 10.1016/j.jmb.2013.09.015. Epub 2013 Sep 20. J Mol Biol. 2013. PMID: 24056173 Free PMC article. Review.
Cited by
-
The use of single-cell RNA-seq to study heterogeneity at varying levels of virus-host interactions.PLoS Pathog. 2024 Jan 18;20(1):e1011898. doi: 10.1371/journal.ppat.1011898. eCollection 2024 Jan. PLoS Pathog. 2024. PMID: 38236826 Free PMC article. Review.
-
Hepatitis C virus RNA is 5'-capped with flavin adenine dinucleotide.Nature. 2023 Jul;619(7971):811-818. doi: 10.1038/s41586-023-06301-3. Epub 2023 Jul 5. Nature. 2023. PMID: 37407817
-
Maximal interferon induction by influenza lacking NS1 is infrequent owing to requirements for replication and export.PLoS Pathog. 2023 Apr 17;19(4):e1010943. doi: 10.1371/journal.ppat.1010943. eCollection 2023 Apr. PLoS Pathog. 2023. PMID: 37068114 Free PMC article.
-
Zika virus noncoding RNA cooperates with the viral protein NS5 to inhibit STAT1 phosphorylation and facilitate viral pathogenesis.Sci Adv. 2022 Dec 2;8(48):eadd8095. doi: 10.1126/sciadv.add8095. Epub 2022 Nov 30. Sci Adv. 2022. PMID: 36449607 Free PMC article.
-
Partial human Janus kinase 1 deficiency predominantly impairs responses to interferon gamma and intracellular control of mycobacteria.Front Immunol. 2022 Sep 9;13:888427. doi: 10.3389/fimmu.2022.888427. eCollection 2022. Front Immunol. 2022. PMID: 36159783 Free PMC article.
References
-
- Apostolou E., Thanos D. Virus infection induces NF-kappaB-dependent interchromosomal associations mediating monoallelic IFN-beta gene expression. Cell. 2008;134(1):85–96. - PubMed
-
- Childs K., Stock N., Ross C., Andrejeva J., Hilton L., Skinner M., Randall R., Goodbourn S. mda-5, but not RIG-I, is a common target for paramyxovirus V proteins. Virology. 2007;359(1):190–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

