Granulocyte-colony stimulating factor reactivates human cytomegalovirus in a latently infected humanized mouse model
- PMID: 20833379
- PMCID: PMC2945885
- DOI: 10.1016/j.chom.2010.08.001
Granulocyte-colony stimulating factor reactivates human cytomegalovirus in a latently infected humanized mouse model
Abstract
Human cytomegalovirus (HCMV) is a significant cause of morbidity and mortality in organ transplant recipients. The use of granulocyte-colony stimulating factor (G-CSF)-mobilized stem cells from HCMV seropositive donors is suggested to double the risk of late-onset HCMV disease and chronic graft-versus-host disease in recipients when compared to conventional bone marrow transplantation with HCMV seropositive donors, although the etiology of the increased risk is unknown. To understand mechanisms of HCMV transmission in patients receiving G-CSF-mobilized blood products, we generated a NOD-scid IL2Rγ(c)(null)-humanized mouse model in which HCMV establishes latent infection in human hematopoietic cells. In this model, G-CSF induces the reactivation of latent HCMV in monocytes/macrophages that have migrated into organ tissues. In addition to establishing a humanized mouse model for systemic and latent HCMV infection, these results suggest that the use of G-CSF mobilized blood products from seropositive donors pose an elevated risk for HCMV transmission to recipients.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
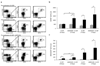
 , pre-mobilized;
, pre-mobilized;  , mobilized. Data represent mean ± s.e.m. (n=4). *, P < 0.05.
, mobilized. Data represent mean ± s.e.m. (n=4). *, P < 0.05.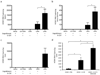

 ), UL83 (pp65,
), UL83 (pp65,  ), and UL146 (
), and UL146 ( ) mRNAs were quantified by real-time PCR of total liver and bone marrow mononuclear cell mRNA. PBS AVG Liver, PBS AVG BM, and G-CSF AVG BM represent the mean values of transcripts for PBS-treated mouse liver tissue, PBS-treated mouse bone marrow mononuclear cells, and G-CSF-treated mouse bone marrow mononuclear cells respectively. b,c, HCMV genomic DNA is present in the bone marrow of G-CSF-mobilized and PBS-treated mice (b) and increases in the liver of HCMV-infected engrafted mice following G-CSF treatment (c). Engrafted mice were infected with HCMV, treated with G-CSF (n=5) or PBS (n=3) for 7 days at 4 weeks post-infection, and sacrificed at 6 weeks post-infection. Total DNA was harvested from organ tissue and analyzed for HCMV genomic DNA by quantitative real time PCR. Data represent mean copies/µg ± s.d. (n=5). *, P < 0.01. d, HCMV gB/gH expression is limited to CD14+ HLA-DR+ monocytes. Engrafted mice were injected IP with HCMV-infected fibroblasts, UV-inactivated HCMV-treated fibroblasts, or mock-infected fibroblasts. At 4 weeks post-infection, mice were administered G-CSF for 7 days and sacrificed. Liver tissue sections were stained with antibodies against huCD14, huHLA-DR, and HCMV gB/gH. 4-color immunofluorescence images of 8 µm liver cryosections were obtained for Hoechst-stained nuclei (blue; all panels), huCD14 (green; left), huHLA-DR (red; middle), and HCMV gB/gH (purple; right). HLA-DR+CD14+ monocytes co-expressing HCMV gB and gH were seen only in HCMV-infected mice treated with G-CSF. Scale bars = 25µm.
) mRNAs were quantified by real-time PCR of total liver and bone marrow mononuclear cell mRNA. PBS AVG Liver, PBS AVG BM, and G-CSF AVG BM represent the mean values of transcripts for PBS-treated mouse liver tissue, PBS-treated mouse bone marrow mononuclear cells, and G-CSF-treated mouse bone marrow mononuclear cells respectively. b,c, HCMV genomic DNA is present in the bone marrow of G-CSF-mobilized and PBS-treated mice (b) and increases in the liver of HCMV-infected engrafted mice following G-CSF treatment (c). Engrafted mice were infected with HCMV, treated with G-CSF (n=5) or PBS (n=3) for 7 days at 4 weeks post-infection, and sacrificed at 6 weeks post-infection. Total DNA was harvested from organ tissue and analyzed for HCMV genomic DNA by quantitative real time PCR. Data represent mean copies/µg ± s.d. (n=5). *, P < 0.01. d, HCMV gB/gH expression is limited to CD14+ HLA-DR+ monocytes. Engrafted mice were injected IP with HCMV-infected fibroblasts, UV-inactivated HCMV-treated fibroblasts, or mock-infected fibroblasts. At 4 weeks post-infection, mice were administered G-CSF for 7 days and sacrificed. Liver tissue sections were stained with antibodies against huCD14, huHLA-DR, and HCMV gB/gH. 4-color immunofluorescence images of 8 µm liver cryosections were obtained for Hoechst-stained nuclei (blue; all panels), huCD14 (green; left), huHLA-DR (red; middle), and HCMV gB/gH (purple; right). HLA-DR+CD14+ monocytes co-expressing HCMV gB and gH were seen only in HCMV-infected mice treated with G-CSF. Scale bars = 25µm.
 ), UL83 (pp65,
), UL83 (pp65,  ), and UL146 (
), and UL146 ( ) mRNAs were quantified by real-time PCR of total liver and bone marrow mononuclear cell mRNA. PBS AVG Liver, PBS AVG BM, and G-CSF AVG BM represent the mean values of transcripts for PBS-treated mouse liver tissue, PBS-treated mouse bone marrow mononuclear cells, and G-CSF-treated mouse bone marrow mononuclear cells respectively. b,c, HCMV genomic DNA is present in the bone marrow of G-CSF-mobilized and PBS-treated mice (b) and increases in the liver of HCMV-infected engrafted mice following G-CSF treatment (c). Engrafted mice were infected with HCMV, treated with G-CSF (n=5) or PBS (n=3) for 7 days at 4 weeks post-infection, and sacrificed at 6 weeks post-infection. Total DNA was harvested from organ tissue and analyzed for HCMV genomic DNA by quantitative real time PCR. Data represent mean copies/µg ± s.d. (n=5). *, P < 0.01. d, HCMV gB/gH expression is limited to CD14+ HLA-DR+ monocytes. Engrafted mice were injected IP with HCMV-infected fibroblasts, UV-inactivated HCMV-treated fibroblasts, or mock-infected fibroblasts. At 4 weeks post-infection, mice were administered G-CSF for 7 days and sacrificed. Liver tissue sections were stained with antibodies against huCD14, huHLA-DR, and HCMV gB/gH. 4-color immunofluorescence images of 8 µm liver cryosections were obtained for Hoechst-stained nuclei (blue; all panels), huCD14 (green; left), huHLA-DR (red; middle), and HCMV gB/gH (purple; right). HLA-DR+CD14+ monocytes co-expressing HCMV gB and gH were seen only in HCMV-infected mice treated with G-CSF. Scale bars = 25µm.
) mRNAs were quantified by real-time PCR of total liver and bone marrow mononuclear cell mRNA. PBS AVG Liver, PBS AVG BM, and G-CSF AVG BM represent the mean values of transcripts for PBS-treated mouse liver tissue, PBS-treated mouse bone marrow mononuclear cells, and G-CSF-treated mouse bone marrow mononuclear cells respectively. b,c, HCMV genomic DNA is present in the bone marrow of G-CSF-mobilized and PBS-treated mice (b) and increases in the liver of HCMV-infected engrafted mice following G-CSF treatment (c). Engrafted mice were infected with HCMV, treated with G-CSF (n=5) or PBS (n=3) for 7 days at 4 weeks post-infection, and sacrificed at 6 weeks post-infection. Total DNA was harvested from organ tissue and analyzed for HCMV genomic DNA by quantitative real time PCR. Data represent mean copies/µg ± s.d. (n=5). *, P < 0.01. d, HCMV gB/gH expression is limited to CD14+ HLA-DR+ monocytes. Engrafted mice were injected IP with HCMV-infected fibroblasts, UV-inactivated HCMV-treated fibroblasts, or mock-infected fibroblasts. At 4 weeks post-infection, mice were administered G-CSF for 7 days and sacrificed. Liver tissue sections were stained with antibodies against huCD14, huHLA-DR, and HCMV gB/gH. 4-color immunofluorescence images of 8 µm liver cryosections were obtained for Hoechst-stained nuclei (blue; all panels), huCD14 (green; left), huHLA-DR (red; middle), and HCMV gB/gH (purple; right). HLA-DR+CD14+ monocytes co-expressing HCMV gB and gH were seen only in HCMV-infected mice treated with G-CSF. Scale bars = 25µm.Comment in
-
G-CSF and CD34+ progenitor cells in hematopoietic grafts: too fertile for human cytomegalovirus.Cell Host Microbe. 2010 Sep 16;8(3):223-4. doi: 10.1016/j.chom.2010.08.009. Cell Host Microbe. 2010. PMID: 20833373
Similar articles
-
HCMV infection of humanized mice after transplantation of G-CSF-mobilized peripheral blood stem cells from HCMV-seropositive donors.Biol Blood Marrow Transplant. 2014 Jan;20(1):132-5. doi: 10.1016/j.bbmt.2013.10.019. Epub 2013 Oct 23. Biol Blood Marrow Transplant. 2014. PMID: 24161922 Free PMC article.
-
Human Cytomegalovirus US28 Ligand Binding Activity Is Required for Latency in CD34+ Hematopoietic Progenitor Cells and Humanized NSG Mice.mBio. 2019 Aug 20;10(4):e01889-19. doi: 10.1128/mBio.01889-19. mBio. 2019. PMID: 31431555 Free PMC article.
-
CD34+ Hematopoietic Progenitor Cell Subsets Exhibit Differential Ability To Maintain Human Cytomegalovirus Latency and Persistence.J Virol. 2021 Jan 13;95(3):e02105-20. doi: 10.1128/JVI.02105-20. Print 2021 Jan 13. J Virol. 2021. PMID: 33177198 Free PMC article.
-
Human cytomegalovirus reactivation in bone-marrow-derived granulocyte/monocyte progenitor cells and mature monocytes.Intervirology. 1999;42(5-6):308-13. doi: 10.1159/000053965. Intervirology. 1999. PMID: 10702711 Review.
-
Human cytomegalovirus infection of human hematopoietic progenitor cells.Leuk Lymphoma. 1999 Mar;33(1-2):1-13. doi: 10.3109/10428199909093720. Leuk Lymphoma. 1999. PMID: 10194116 Review.
Cited by
-
Viral and host network analysis of the human cytomegalovirus transcriptome in latency.bioRxiv [Preprint]. 2024 May 21:2024.05.21.594597. doi: 10.1101/2024.05.21.594597. bioRxiv. 2024. PMID: 38826434 Free PMC article. Preprint.
-
Proximity-dependent mapping of the HCMV US28 interactome identifies RhoGEF signaling as a requirement for efficient viral reactivation.PLoS Pathog. 2023 Oct 2;19(10):e1011682. doi: 10.1371/journal.ppat.1011682. eCollection 2023 Oct. PLoS Pathog. 2023. PMID: 37782657 Free PMC article.
-
Stabilization of the human cytomegalovirus UL136p33 reactivation determinant overcomes the requirement for UL135 for replication in hematopoietic cells.J Virol. 2023 Aug 31;97(8):e0014823. doi: 10.1128/jvi.00148-23. Epub 2023 Aug 11. J Virol. 2023. PMID: 37565749 Free PMC article.
-
Mouse Models for Human Herpesviruses.Pathogens. 2023 Jul 19;12(7):953. doi: 10.3390/pathogens12070953. Pathogens. 2023. PMID: 37513800 Free PMC article. Review.
-
Hematopoietic stem cells and betaherpesvirus latency.Front Cell Infect Microbiol. 2023 Jun 6;13:1189805. doi: 10.3389/fcimb.2023.1189805. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37346032 Free PMC article. Review.
References
-
- Anderson D, DeFor T, Burns L, McGlave P, Miller J, Wagner J, Weisdorf D. A comparison of related donor peripheral blood and bone marrow transplants: importance of late-onset chronic graft-versus-host disease and infections. Biol Blood Marrow Transplant. 2003;9:52–59. - PubMed
-
- Asano-Mori Y, Kanda Y, Oshima K, Kako S, Shinohara A, Nakasone H, Sato H, Watanabe T, Hosoya N, Izutsu K, et al. Clinical features of late cytomegalovirus infection after hematopoietic stem cell transplantation. Int J Hematol. 2008;87:310–318. - PubMed
-
- Bidanset DJ, Rybak RJ, Hartline CB, Kern ER. Replication of human cytomegalovirus in severe combined immunodeficient mice implanted with human retinal tissue. J Infect Dis. 2001;184:192–195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 AI071940/AI/NIAID NIH HHS/United States
- R01 HL065754-08/HL/NHLBI NIH HHS/United States
- R01 AI039416-13/AI/NIAID NIH HHS/United States
- HL083194/HL/NHLBI NIH HHS/United States
- R01 HL083194/HL/NHLBI NIH HHS/United States
- R01 HL071695/HL/NHLBI NIH HHS/United States
- R01 HL069133/HL/NHLBI NIH HHS/United States
- R01 AI021640-26/AI/NIAID NIH HHS/United States
- R01 HL077818-04/HL/NHLBI NIH HHS/United States
- R01 AI021640/AI/NIAID NIH HHS/United States
- R01 AI039416/AI/NIAID NIH HHS/United States
- R01 HL069133-07/HL/NHLBI NIH HHS/United States
- R33 AI071940-06/AI/NIAID NIH HHS/United States
- R01 HL083194-05/HL/NHLBI NIH HHS/United States
- R01 AI073146/AI/NIAID NIH HHS/United States
- R01 AI073146-05/AI/NIAID NIH HHS/United States
- HL88603/HL/NHLBI NIH HHS/United States
- R33 AI071940/AI/NIAID NIH HHS/United States
- R01 HL088603/HL/NHLBI NIH HHS/United States
- HL65754/HL/NHLBI NIH HHS/United States
- AI71940/AI/NIAID NIH HHS/United States
- AI21640/AI/NIAID NIH HHS/United States
- AI39416/AI/NIAID NIH HHS/United States
- AI73146/AI/NIAID NIH HHS/United States
- R01 HL077818/HL/NHLBI NIH HHS/United States
- HL069133/HL/NHLBI NIH HHS/United States
- R01 HL088603-04/HL/NHLBI NIH HHS/United States
- R37 AI021640/AI/NIAID NIH HHS/United States
- HL077818/HL/NHLBI NIH HHS/United States
- R01 HL065754/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

