Interaction of hypoxia-inducible factor-1α and Notch signaling regulates medulloblastoma precursor proliferation and fate
- PMID: 20827750
- PMCID: PMC3474900
- DOI: 10.1002/stem.518
Interaction of hypoxia-inducible factor-1α and Notch signaling regulates medulloblastoma precursor proliferation and fate
Abstract
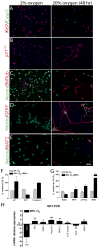
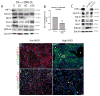
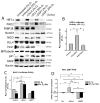
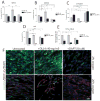
Medulloblastoma (MDB) is the most common brain malignancy of childhood. It is currently thought that MDB arises from aberrantly functioning stem cells in the cerebellum that fail to maintain proper control of self-renewal. Additionally, it has been reported that MDB cells display higher endogenous Notch signaling activation, known to promote the survival and proliferation of neoplastic neural stem cells and to inhibit their differentiation. Although interaction between hypoxia-inducible factor-1α (HIF-1α) and Notch signaling is required to maintain normal neural precursors in an undifferentiated state, an interaction has not been identified in MDB. Here, we investigate whether hypoxia, through HIF-1α stabilization, modulates Notch1 signaling in primary MDB-derived cells. Our results indicate that MDB-derived precursor cells require hypoxic conditions for in vitro expansion, whereas acute exposure to 20% oxygen induces tumor cell differentiation and death through inhibition of Notch signaling. Importantly, stimulating Notch1 activation with its ligand Dll4 under hypoxic conditions leads to expansion of MDB-derived CD133(+) and nestin(+) precursors, suggesting a regulatory effect on stem cells. In contrast, MDB cells undergo neuronal differentiation when treated with γ-secretase inhibitor, which prevents Notch activation. These results suggest that hypoxia, by maintaining Notch1 in its active form, preserves MDB stem cell viability and expansion.
Copyright © 2010 AlphaMed Press.
Conflict of interest statement
The authors indicate no potential conflicts of interest.
Figures


Similar articles
-
Methylprednisolone inhibits the proliferation and affects the differentiation of rat spinal cord-derived neural progenitor cells cultured in low oxygen conditions by inhibiting HIF-1α and Hes1 in vitro.Int J Mol Med. 2014 Sep;34(3):788-95. doi: 10.3892/ijmm.2014.1835. Epub 2014 Jul 3. Int J Mol Med. 2014. PMID: 24992925
-
Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1alpha.Oncogene. 2009 Nov 12;28(45):3949-59. doi: 10.1038/onc.2009.252. Epub 2009 Aug 31. Oncogene. 2009. PMID: 19718046
-
Hypoxia requires notch signaling to maintain the undifferentiated cell state.Dev Cell. 2005 Nov;9(5):617-28. doi: 10.1016/j.devcel.2005.09.010. Dev Cell. 2005. PMID: 16256737
-
Stem cell markers in gliomas.Neurochem Res. 2008 Dec;33(12):2407-15. doi: 10.1007/s11064-008-9723-8. Epub 2008 May 21. Neurochem Res. 2008. PMID: 18493853 Review.
-
Hypoxia-inducible factors, stem cells, and cancer.Cell. 2007 May 4;129(3):465-72. doi: 10.1016/j.cell.2007.04.019. Cell. 2007. PMID: 17482542 Free PMC article. Review.
Cited by
-
The biological kinship of hypoxia with CSC and EMT and their relationship with deregulated expression of miRNAs and tumor aggressiveness.Biochim Biophys Acta. 2012 Dec;1826(2):272-96. doi: 10.1016/j.bbcan.2012.04.008. Epub 2012 May 10. Biochim Biophys Acta. 2012. PMID: 22579961 Free PMC article. Review.
-
Overcoming Treatment Resistance in Medulloblastoma: Underlying Mechanisms and Potential Strategies.Cancers (Basel). 2024 Jun 18;16(12):2249. doi: 10.3390/cancers16122249. Cancers (Basel). 2024. PMID: 38927954 Free PMC article. Review.
-
The roles of hypoxia-inducible factors in regulating neural stem cells migration to glioma stem cells and determinating their fates.Neurochem Res. 2012 Dec;37(12):2659-66. doi: 10.1007/s11064-012-0879-x. Epub 2012 Sep 19. Neurochem Res. 2012. PMID: 22991140 Review.
-
Notch signaling change in pulmonary vascular remodeling in rats with pulmonary hypertension and its implication for therapeutic intervention.PLoS One. 2012;7(12):e51514. doi: 10.1371/journal.pone.0051514. Epub 2012 Dec 12. PLoS One. 2012. PMID: 23251561 Free PMC article.
-
A Tale of Two: When Neural Stem Cells Encounter Hypoxia.Cell Mol Neurobiol. 2023 Jul;43(5):1799-1816. doi: 10.1007/s10571-022-01293-6. Epub 2022 Oct 29. Cell Mol Neurobiol. 2023. PMID: 36308642 Free PMC article. Review.
References
-
- Partap S, Curran EK, Propp JM, Le GM, Sainani KL, Fisher PG. Medulloblastoma incidence has not changed over time: a CBTRUS study. J Pediatr Hematol Oncol. 2009 Dec;31(12):970–971. - PubMed
-
- Fan X, Matsui W, Khaki L, et al. Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 2006 Aug 1;66(15):7445–7452. - PubMed
-
- Fan X, Mikolaenko I, Elhassan I, et al. Notch1 and notch2 have opposite effects on embryonal brain tumor growth. Cancer Res. 2004 Nov 1;64(21):7787–7793. - PubMed
-
- Hallahan AR, Pritchard JI, Hansen S, et al. The SmoA1 mouse model reveals that notch signaling is critical for the growth and survival of sonic hedgehog-induced medulloblastomas. Cancer Res. 2004 Nov 1;64(21):7794–7800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

