Annexin A2 mediates up-regulation of NF-κB, β-catenin, and stem cell in response to progastrin in mice and HEK-293 cells
- PMID: 20826156
- PMCID: PMC3031715
- DOI: 10.1053/j.gastro.2010.08.054
Annexin A2 mediates up-regulation of NF-κB, β-catenin, and stem cell in response to progastrin in mice and HEK-293 cells
Abstract
Background & aims: Prograstrin induces proliferation in colon crypts by activating p65nuclear factor-κB (NF-κB) (p65) and β-catenin. We investigated whether Annexin A2 (AnxA2), a progastrin receptor, activates NF-κB and β-catenin in vivo.
Methods: ANXA2-null (ANXA2(-/-)) and wild-type (ANXA2(+/+)) mice were studied, along with clones of progastrin-responsive HEK-293 cells that stably expressed full-length progastrin (HEK-mGAS) or an empty vector (HEK-C). Small interfering RNA was used to down-regulate AnxA2, p65NF-κB, and β-catenin in cells.
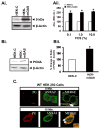
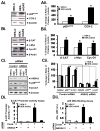
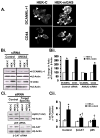
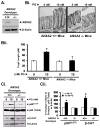
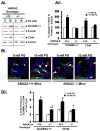
Results: Proliferation and activation of p65 and β-catenin increased significantly in HEK-mGAS compared with HEK-C clones. HEK-mGAS cells had a 2- to 4-fold increase in relative levels of c-Myc, cyclooxygenase (COX)-2, CyclinD1, double cortin CAM kinase-like 1 (DCAMKL+1), and CD44, compared with HEK-C clones. Down-regulation of AnxA2 in HEK-mGAS clones reduced activation of NF-κB and β-catenin, as well as levels of DCAMKL+1. Surprisingly, down-regulation of β-catenin had no effect on activation of p65NF-κB, whereas down-regulation of p65 significantly reduced activation of β-catenin in HEK-mGAS clones. Loss of either p65 or β-catenin significantly reduced proliferation of HEK-mGAS clones, indicating that both factors are required for the proliferative effects of progastrin. Lengths of colon crypts and levels of p65, β-catenin, DCAMKL+1, and CD44 were significantly higher in ANXA2(+/+) mice compared with either ANXA2(-/-) mice given progastrin or ANXA2(+/+) and ANXA2(-/-) mice given saline.
Conclusions: AnxA2 expression is required for the biologic effects of progastrin in vivo and in vitro and mediates the stimulatory effect of progastrin on p65NF-κ, β-catenin, and the putative stem cell markers DCAMKL+1 and CD44. AnxA2 might therefore mediate the hyperproliferative and cocarcinogenic effects of progastrin.
Copyright © 2011 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

Similar articles
-
Progastrin overexpression imparts tumorigenic/metastatic potential to embryonic epithelial cells: phenotypic differences between transformed and nontransformed stem cells.Int J Cancer. 2012 Oct 1;131(7):E1088-99. doi: 10.1002/ijc.27615. Epub 2012 May 17. Int J Cancer. 2012. PMID: 22532325 Free PMC article.
-
Functional cross-talk between beta-catenin and NFkappaB signaling pathways in colonic crypts of mice in response to progastrin.J Biol Chem. 2009 Aug 14;284(33):22274-22284. doi: 10.1074/jbc.M109.020941. Epub 2009 Jun 4. J Biol Chem. 2009. PMID: 19497850 Free PMC article.
-
Antiapoptotic effects of progastrin on pancreatic cancer cells are mediated by sustained activation of nuclear factor-{kappa}B.Cancer Res. 2007 Aug 1;67(15):7266-74. doi: 10.1158/0008-5472.CAN-07-1206. Cancer Res. 2007. PMID: 17671195
-
Role of Annexin-II in GI cancers: interaction with gastrins/progastrins.Cancer Lett. 2007 Jul 8;252(1):19-35. doi: 10.1016/j.canlet.2006.11.012. Epub 2006 Dec 22. Cancer Lett. 2007. PMID: 17188424 Free PMC article. Review.
-
Impact of Annexin A2 on virus life cycles.Virus Res. 2024 Jul;345:199384. doi: 10.1016/j.virusres.2024.199384. Epub 2024 May 7. Virus Res. 2024. PMID: 38702018 Free PMC article. Review.
Cited by
-
Epigenetic regulation of human DCLK-1 gene during colon-carcinogenesis: clinical and mechanistic implications.Stem Cell Investig. 2016 Sep 28;3:51. doi: 10.21037/sci.2016.09.07. eCollection 2016. Stem Cell Investig. 2016. PMID: 27777940 Free PMC article.
-
FOXQ1 promotes the osteogenic differentiation of bone mesenchymal stem cells via Wnt/β-catenin signaling by binding with ANXA2.Stem Cell Res Ther. 2020 Sep 17;11(1):403. doi: 10.1186/s13287-020-01928-9. Stem Cell Res Ther. 2020. PMID: 32943107 Free PMC article.
-
Progastrin-induced secretion of insulin-like growth factor 2 from colonic myofibroblasts stimulates colonic epithelial proliferation in mice.Gastroenterology. 2013 Jul;145(1):197-208.e3. doi: 10.1053/j.gastro.2013.03.012. Epub 2013 Mar 19. Gastroenterology. 2013. PMID: 23523669 Free PMC article.
-
Progastrin Peptides Increase the Risk of Developing Colonic Tumors: Impact on Colonic Stem Cells.Curr Colorectal Cancer Rep. 2012 Dec;8(4):277-289. doi: 10.1007/s11888-012-0144-3. Curr Colorectal Cancer Rep. 2012. PMID: 23226720 Free PMC article.
-
S100A11 functions as novel oncogene in glioblastoma via S100A11/ANXA2/NF-κB positive feedback loop.J Cell Mol Med. 2019 Oct;23(10):6907-6918. doi: 10.1111/jcmm.14574. Epub 2019 Aug 20. J Cell Mol Med. 2019. PMID: 31430050 Free PMC article.
References
-
- Rengifo-Cam W, Singh P. Role of progastrins and gastrins and their receptors in GI and pancreatic cancers: targets for treatment. Curr Pharm Des. 2004;10:2345–2358. - PubMed
-
- Grabowska AM, Watson SA. Role of gastrin peptides in carcinogenesis. Cancer Lett. 2007;257:1–15. - PubMed
-
- Baldwin GS, Hollande F, Yang Z, et al. Biologically active recombinant human progastrin contains a tightly bound calcium ion. J Biol Chem. 2001;276:7791–7796. - PubMed
-
- Singh P, Lu X, Cobb S, et al. Progastrin1–80 stimulates growth of intestinal epithelial cells in vitro via high-affinity binding sites. Am J Physiol. 2003;284:G328–G339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL090895-04/HL/NHLBI NIH HHS/United States
- R01 CA097959/CA/NCI NIH HHS/United States
- P01 HL046403/HL/NHLBI NIH HHS/United States
- P01 HL046403-20/HL/NHLBI NIH HHS/United States
- R01 HL042493-18/HL/NHLBI NIH HHS/United States
- HL 046403/HL/NHLBI NIH HHS/United States
- R01 HL090895/HL/NHLBI NIH HHS/United States
- HL 090895/HL/NHLBI NIH HHS/United States
- CA114264/CA/NCI NIH HHS/United States
- R01 CA114264/CA/NCI NIH HHS/United States
- HL 042493/HL/NHLBI NIH HHS/United States
- CA97959/CA/NCI NIH HHS/United States
- R01 HL042493/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

