NANOG reporter cell lines generated by gene targeting in human embryonic stem cells
- PMID: 20824089
- PMCID: PMC2932718
- DOI: 10.1371/journal.pone.0012533
NANOG reporter cell lines generated by gene targeting in human embryonic stem cells
Abstract
Background: Pluripotency and self-renewal of human embryonic stem cells (hESCs) is mediated by a complex interplay between extra- and intracellular signaling pathways, which regulate the expression of pluripotency-specific transcription factors. The homeodomain transcription factor NANOG plays a central role in maintaining hESC pluripotency, but the precise role and regulation of NANOG are not well defined.
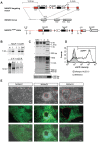
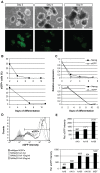
Methodology/principal findings: To facilitate the study of NANOG expression and regulation in viable hESC cultures, we generated fluorescent NANOG reporter cell lines by gene targeting in hESCs. In these reporter lines, the fluorescent reporter gene was co-expressed with endogenous NANOG and responded to experimental induction or repression of the NANOG promoter with appropriate changes in expression levels. Furthermore, NANOG reporter lines facilitated the separation of hESC populations based on NANOG expression levels and their subsequent characterization. Gene expression arrays on isolated hESC subpopulations revealed genes with differential expression in NANOG(high) and NANOG(low) hESCs, providing candidates for NANOG downstream targets hESCs.
Conclusion/significance: The newly derived NANOG reporter hESC lines present novel tools to visualize NANOG expression in viable hESCs. In future applications, these reporter lines can be used to elucidate the function and regulation of NANOG in pluripotent hESCs.
Conflict of interest statement
Figures

Similar articles
-
KLF4 and PBX1 directly regulate NANOG expression in human embryonic stem cells.Stem Cells. 2009 Sep;27(9):2114-25. doi: 10.1002/stem.143. Stem Cells. 2009. PMID: 19522013
-
L1TD1 is a marker for undifferentiated human embryonic stem cells.PLoS One. 2011 Apr 29;6(4):e19355. doi: 10.1371/journal.pone.0019355. PLoS One. 2011. PMID: 21559406 Free PMC article.
-
Brief report: L1 cell adhesion molecule, a novel surface molecule of human embryonic stem cells, is essential for self-renewal and pluripotency.Stem Cells. 2011 Dec;29(12):2094-9. doi: 10.1002/stem.754. Stem Cells. 2011. PMID: 21957033
-
Nanog and transcriptional networks in embryonic stem cell pluripotency.Cell Res. 2007 Jan;17(1):42-9. doi: 10.1038/sj.cr.7310125. Cell Res. 2007. PMID: 17211451 Review.
-
Homologous recombination in human embryonic stem cells: a tool for advancing cell therapy and understanding and treating human disease.Clin Transl Sci. 2011 Aug;4(4):298-305. doi: 10.1111/j.1752-8062.2011.00281.x. Clin Transl Sci. 2011. PMID: 21884519 Free PMC article. Review.
Cited by
-
A critical review on therapeutic approaches of CRISPR-Cas9 in diabetes mellitus.Naunyn Schmiedebergs Arch Pharmacol. 2023 Dec;396(12):3459-3481. doi: 10.1007/s00210-023-02631-1. Epub 2023 Jul 31. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 37522916 Review.
-
BMP4 signaling directs primitive endoderm-derived XEN cells to an extraembryonic visceral endoderm identity.Dev Biol. 2012 Jan 15;361(2):245-62. doi: 10.1016/j.ydbio.2011.10.015. Epub 2011 Oct 15. Dev Biol. 2012. PMID: 22051107 Free PMC article.
-
Find and replace: editing human genome in pluripotent stem cells.Protein Cell. 2011 Dec;2(12):950-6. doi: 10.1007/s13238-011-1132-0. Epub 2011 Dec 15. Protein Cell. 2011. PMID: 22173708 Free PMC article. Review.
-
Turning straw into gold: directing cell fate for regenerative medicine.Nat Rev Genet. 2011 Apr;12(4):243-52. doi: 10.1038/nrg2938. Epub 2011 Mar 9. Nat Rev Genet. 2011. PMID: 21386864 Review.
-
Single-cell analysis reveals that expression of nanog is biallelic and equally variable as that of other pluripotency factors in mouse ESCs.Cell Stem Cell. 2013 Jul 3;13(1):23-9. doi: 10.1016/j.stem.2013.04.019. Cell Stem Cell. 2013. PMID: 23827708 Free PMC article.
References
-
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. - PubMed
-
- Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. - PubMed
-
- Zaehres H, Lensch MW, Daheron L, Stewart SA, Itskovitz-Eldor J, et al. High-efficiency RNA interference in human embryonic stem cells. Stem Cells. 2005;23:299–305. - PubMed
-
- Hyslop L, Stojkovic M, Armstrong L, Walter T, Stojkovic P, et al. Downregulation of NANOG induces differentiation of human embryonic stem cells to extraembryonic lineages. Stem Cells. 2005;23:1035–1043. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

