Mutations in mouse Aspm (abnormal spindle-like microcephaly associated) cause not only microcephaly but also major defects in the germline
- PMID: 20823249
- PMCID: PMC2944708
- DOI: 10.1073/pnas.1010494107
Mutations in mouse Aspm (abnormal spindle-like microcephaly associated) cause not only microcephaly but also major defects in the germline
Abstract
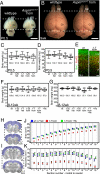
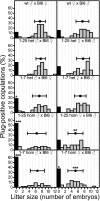
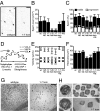
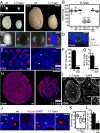
Mutations in ASPM (abnormal spindle-like microcephaly associated) cause primary microcephaly in humans, a disorder characterized by a major reduction in brain size in the apparent absence of nonneurological anomalies. The function of the Aspm protein in neural progenitor cell expansion, as well as its localization to the mitotic spindle and midbody, suggest that it regulates brain development by a cell division-related mechanism. Furthermore, evidence that positive selection affected ASPM during primate evolution has led to suggestions that such a function changed during primate evolution. Here, we report that in Aspm mutant mice, truncated Aspm proteins similar to those causing microcephaly in humans fail to localize to the midbody during M-phase and cause mild microcephaly. A human ASPM transgene rescues this phenotype but, interestingly, does not cause a gain of function. Strikingly, truncated Aspm proteins also cause a massive loss of germ cells, resulting in a severe reduction in testis and ovary size accompanied by reduced fertility. These germline effects, too, are fully rescued by the human ASPM transgene, indicating that ASPM is functionally similar in mice and humans. Our findings broaden the spectrum of phenotypic effects of ASPM mutations and raise the possibility that positive selection of ASPM during primate evolution reflects its function in the germline.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Aspm knockout ferret reveals an evolutionary mechanism governing cerebral cortical size.Nature. 2018 Apr;556(7701):370-375. doi: 10.1038/s41586-018-0035-0. Epub 2018 Apr 11. Nature. 2018. PMID: 29643508 Free PMC article.
-
Loss of abnormal spindle-like, microcephaly-associated (Aspm) disrupts female folliculogenesis in mice during maturation and aging.Reprod Biol. 2022 Sep;22(3):100673. doi: 10.1016/j.repbio.2022.100673. Epub 2022 Jul 25. Reprod Biol. 2022. PMID: 35901620
-
Disruption of Aspm causes microcephaly with abnormal neuronal differentiation.Brain Dev. 2014 Sep;36(8):661-9. doi: 10.1016/j.braindev.2013.10.006. Epub 2013 Nov 9. Brain Dev. 2014. PMID: 24220505
-
The Multiple Mitotic Roles of the ASPM Orthologous Proteins: Insight into the Etiology of ASPM-Dependent Microcephaly.Cells. 2023 Mar 16;12(6):922. doi: 10.3390/cells12060922. Cells. 2023. PMID: 36980263 Free PMC article. Review.
-
Evolution of primary microcephaly genes and the enlargement of primate brains.Curr Opin Genet Dev. 2005 Jun;15(3):241-8. doi: 10.1016/j.gde.2005.04.009. Curr Opin Genet Dev. 2005. PMID: 15917198 Review.
Cited by
-
Functional divergence of the brain-size regulating gene MCPH1 during primate evolution and the origin of humans.BMC Biol. 2013 May 22;11:62. doi: 10.1186/1741-7007-11-62. BMC Biol. 2013. PMID: 23697381 Free PMC article.
-
Deficient adaptation to centrosome duplication defects in neural progenitors causes microcephaly and subcortical heterotopias.JCI Insight. 2021 Aug 23;6(16):e146364. doi: 10.1172/jci.insight.146364. JCI Insight. 2021. PMID: 34237032 Free PMC article.
-
In vitro modeling for inherited neurological diseases using induced pluripotent stem cells: from 2D to organoid.Arch Pharm Res. 2020 Sep;43(9):877-889. doi: 10.1007/s12272-020-01260-z. Epub 2020 Aug 5. Arch Pharm Res. 2020. PMID: 32761309 Review.
-
Automated Immunofluorescence Staining for Analysis of Mitotic Stages and Division Orientation in Brain Sections.Methods Mol Biol. 2023;2583:63-79. doi: 10.1007/978-1-0716-2752-5_7. Methods Mol Biol. 2023. PMID: 36418726
-
Using C. elegans to decipher the cellular and molecular mechanisms underlying neurodevelopmental disorders.Mol Neurobiol. 2013 Dec;48(3):465-89. doi: 10.1007/s12035-013-8434-6. Epub 2013 Mar 14. Mol Neurobiol. 2013. PMID: 23494747 Review.
References
-
- Kriegstein A, Noctor S, Martínez-Cerdeño V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat Rev Neurosci. 2006;7:883–890. - PubMed
-
- Fish JL, Dehay C, Kennedy H, Huttner WB. Making bigger brains—the evolution of neural-progenitor-cell division. J Cell Sci. 2008;121:2783–2793. - PubMed
-
- Woods CG. Human microcephaly. Curr Opin Neurobiol. 2004;14:112–117. - PubMed
-
- Bond J, et al. ASPM is a major determinant of cerebral cortical size. Nat Genet. 2002;32:316–320. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

