Shared dependence on the DNA-binding factor TOX for the development of lymphoid tissue-inducer cell and NK cell lineages
- PMID: 20818394
- PMCID: PMC2943551
- DOI: 10.1038/ni.1930
Shared dependence on the DNA-binding factor TOX for the development of lymphoid tissue-inducer cell and NK cell lineages
Abstract
TOX is a DNA-binding factor required for development of CD4(+) T cells, natural killer T cells and regulatory T cells. Here we document that both natural killer (NK) cell development and lymphoid tissue organogenesis were also inhibited in the absence of TOX. We found that the development of lymphoid tissue-inducer cells, a rare subset of specialized cells that has an integral role in lymphoid tissue organogenesis, required TOX. Tox was upregulated considerably in immature NK cells in the bone marrow, consistent with the loss of mature NK cells in the absence of this nuclear protein. Thus, many cell lineages of the immune system share a TOX-dependent step for development.
Figures
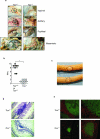

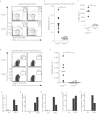
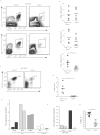


Comment in
-
The innate side of TOX.Nat Immunol. 2010 Oct;11(10):885-6. doi: 10.1038/ni1010-885. Nat Immunol. 2010. PMID: 20856218 No abstract available.
Similar articles
-
The Role of TOX in the Development of Innate Lymphoid Cells.Mediators Inflamm. 2015;2015:243868. doi: 10.1155/2015/243868. Epub 2015 Oct 18. Mediators Inflamm. 2015. PMID: 26556952 Free PMC article. Review.
-
High-mobility group box (TOX) antibody a useful tool for the identification of B and T cell subpopulations.PLoS One. 2020 Feb 27;15(2):e0229743. doi: 10.1371/journal.pone.0229743. eCollection 2020. PLoS One. 2020. PMID: 32106280 Free PMC article.
-
TOX regulates the differentiation of human natural killer cells from hematopoietic stem cells in vitro.Immunol Lett. 2011 Apr 30;136(1):29-36. doi: 10.1016/j.imlet.2010.11.008. Epub 2010 Nov 30. Immunol Lett. 2011. PMID: 21126536
-
The development of innate lymphoid cells requires TOX-dependent generation of a common innate lymphoid cell progenitor.Nat Immunol. 2015 Jun;16(6):599-608. doi: 10.1038/ni.3168. Epub 2015 Apr 27. Nat Immunol. 2015. PMID: 25915732 Free PMC article.
-
Lymphoid tissue inducer-A divergent member of the ILC family.Cytokine Growth Factor Rev. 2018 Aug;42:5-12. doi: 10.1016/j.cytogfr.2018.02.004. Epub 2018 Feb 13. Cytokine Growth Factor Rev. 2018. PMID: 29454785 Free PMC article. Review.
Cited by
-
Intrathymic programming of effector fates in three molecularly distinct γδ T cell subtypes.Nat Immunol. 2012 Apr 1;13(5):511-8. doi: 10.1038/ni.2247. Nat Immunol. 2012. PMID: 22473038 Free PMC article.
-
Cytokine crowdsourcing: multicellular production of TH17-associated cytokines.J Leukoc Biol. 2015 Mar;97(3):499-510. doi: 10.1189/jlb.3RU0814-386R. Epub 2014 Dec 29. J Leukoc Biol. 2015. PMID: 25548251 Free PMC article. Review.
-
A network of high-mobility group box transcription factors programs innate interleukin-17 production.Immunity. 2013 Apr 18;38(4):681-93. doi: 10.1016/j.immuni.2013.01.010. Epub 2013 Apr 4. Immunity. 2013. PMID: 23562159 Free PMC article.
-
Transcriptional regulation of innate lymphoid cell fate.Nat Rev Immunol. 2015 Jul;15(7):415-28. doi: 10.1038/nri3855. Epub 2015 Jun 12. Nat Rev Immunol. 2015. PMID: 26065585 Review.
-
Notch, Id2, and RORγt sequentially orchestrate the fetal development of lymphoid tissue inducer cells.J Exp Med. 2012 Apr 9;209(4):729-40. doi: 10.1084/jem.20111594. Epub 2012 Mar 19. J Exp Med. 2012. PMID: 22430492 Free PMC article.
References
-
- Koni PA, et al. Distinct roles in lymphoid organogenesis for lymphotoxins alpha and beta revealed in lymphotoxin beta-deficient mice. Immunity. 1997;6:491–500. - PubMed
-
- De Togni P, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–707. - PubMed
-
- Ansel KM, et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–314. - PubMed
-
- Cao X, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

