The role of cysteine oxidation in DJ-1 function and dysfunction
- PMID: 20812780
- PMCID: PMC3110098
- DOI: 10.1089/ars.2010.3481
The role of cysteine oxidation in DJ-1 function and dysfunction
Abstract
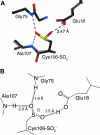
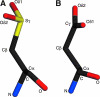
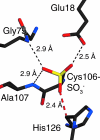
DJ-1 is a member of the large and functionally diverse DJ-1/PfpI superfamily and has homologs in nearly all organisms. Because of its connection to parkinsonism and cancer, human DJ-1 has been intensely studied for over a decade. The current view is that DJ-1 is a multifunctional oxidative stress response protein that defends cells against reactive oxygen species and mitochondrial damage, although the details of its biochemical function remain unclear. A conserved cysteine residue in DJ-1 (Cys106) is both functionally essential and subject to oxidation to the cysteine-sulfinate and cysteine-sulfonate. Consequently, the oxidative modification of Cys106 has been proposed to allow DJ-1 to act as a sensor of cellular redox homeostasis and to participate in cytoprotective signaling pathways in the cell. This review explores the current evidence for the role of cysteine oxidation in DJ-1 function, with emphasis on emerging models for how oxidative modification may regulate DJ-1's protective function and also contribute to dysfunction and disease.
Figures


Similar articles
-
Conservation of oxidative protein stabilization in an insect homologue of parkinsonism-associated protein DJ-1.Biochemistry. 2012 May 8;51(18):3799-807. doi: 10.1021/bi3003296. Epub 2012 Apr 24. Biochemistry. 2012. PMID: 22515803 Free PMC article.
-
Formation of a stabilized cysteine sulfinic acid is critical for the mitochondrial function of the parkinsonism protein DJ-1.J Biol Chem. 2009 Mar 6;284(10):6476-85. doi: 10.1074/jbc.M806599200. Epub 2009 Jan 5. J Biol Chem. 2009. PMID: 19124468 Free PMC article.
-
Use of cysteine-reactive cross-linkers to probe conformational flexibility of human DJ-1 demonstrates that Glu18 mutations are dimers.J Neurochem. 2014 Sep;130(6):839-53. doi: 10.1111/jnc.12763. Epub 2014 Jun 19. J Neurochem. 2014. PMID: 24832775 Free PMC article.
-
DJ-1 and prevention of oxidative stress in Parkinson's disease and other age-related disorders.Free Radic Biol Med. 2009 Nov 15;47(10):1354-61. doi: 10.1016/j.freeradbiomed.2009.08.003. Epub 2009 Aug 14. Free Radic Biol Med. 2009. PMID: 19686841 Review.
-
Small Substrate or Large? Debate Over the Mechanism of Glycation Adduct Repair by DJ-1.Cell Chem Biol. 2020 Sep 17;27(9):1117-1123. doi: 10.1016/j.chembiol.2020.07.016. Epub 2020 Aug 11. Cell Chem Biol. 2020. PMID: 32783963 Free PMC article. Review.
Cited by
-
DJ-1-dependent regulation of oxidative stress in the retinal pigment epithelium (RPE).PLoS One. 2013 Jul 2;8(7):e67983. doi: 10.1371/journal.pone.0067983. Print 2013. PLoS One. 2013. PMID: 23844142 Free PMC article.
-
Modelling Parkinson's Disease: iPSCs towards Better Understanding of Human Pathology.Brain Sci. 2021 Mar 14;11(3):373. doi: 10.3390/brainsci11030373. Brain Sci. 2021. PMID: 33799491 Free PMC article. Review.
-
Thiol-redox signaling, dopaminergic cell death, and Parkinson's disease.Antioxid Redox Signal. 2012 Dec 15;17(12):1764-84. doi: 10.1089/ars.2011.4501. Epub 2012 May 3. Antioxid Redox Signal. 2012. PMID: 22369136 Free PMC article. Review.
-
Mechanistic Nanotherapeutic Approach Based on siRNA-Mediated DJ-1 Protein Suppression for Platinum-Resistant Ovarian Cancer.Mol Pharm. 2016 Jun 6;13(6):2070-83. doi: 10.1021/acs.molpharmaceut.6b00205. Epub 2016 May 26. Mol Pharm. 2016. PMID: 27170529 Free PMC article.
-
DJ-1 Modulates Nuclear Erythroid 2-Related Factor-2-Mediated Protection in Human Primary Alveolar Type II Cells in Smokers.Am J Respir Cell Mol Biol. 2016 Sep;55(3):439-49. doi: 10.1165/rcmb.2015-0304OC. Am J Respir Cell Mol Biol. 2016. PMID: 27093578 Free PMC article.
References
-
- Alsøe L. Stacy JE. Fosså A. Funderud S. Brekke OH. Gaudernack G. Identification of prostate cancer antigens by automated high-throughput filter immunoscreening. J Immunol Methods. 2008;330:12–23. - PubMed
-
- Annesi G. Savettieri G. Pugliese P. D'Amelio M. Tarantino P. Ragonese P. La Bella V. Piccoli T. Civitelli D. Annesi F. Fierro B. Piccoli F. Arabia G. Caracciolo M. Cirò Candiano IC. Quattrone A. DJ-1 mutations and parkinsonism-dementia-amyotrophic lateral sclerosis complex. Ann Neurol. 2005;58:803–807. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

