Involvement of p114-RhoGEF and Lfc in Wnt-3a- and dishevelled-induced RhoA activation and neurite retraction in N1E-115 mouse neuroblastoma cells
- PMID: 20810787
- PMCID: PMC2954123
- DOI: 10.1091/mbc.E10-02-0095
Involvement of p114-RhoGEF and Lfc in Wnt-3a- and dishevelled-induced RhoA activation and neurite retraction in N1E-115 mouse neuroblastoma cells
Abstract
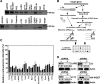
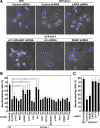
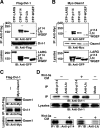
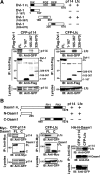
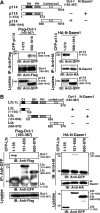
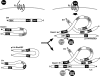
The Wnt-induced planar cell polarity (PCP) signaling pathway is essential for polarized cell migration and morphogenesis. Dishevelled (Dvl) and its binding protein Daam1 mediate RhoA activation in this pathway. WGEF, a member of the Rho-guanine nucleotide exchange factor (Rho-GEF) family, was shown to play a role in Wnt-induced RhoA activation in Xenopus embryos. However, it has remained unknown which member(s) of a Rho-GEF family are involved in Wnt/Dvl-induced RhoA activation in mammalian cells. Here we identified p114-RhoGEF and Lfc (also called GEF-H1) as the Rho-GEFs responsible for Wnt-3a-induced RhoA activation in N1E-115 mouse neuroblastoma cells. We screened for Rho-GEF-silencing short-hairpin RNAs (shRNAs) that are capable of suppressing Dvl-induced neurite retraction in N1E-115 cells and found that p114-RhoGEF and Lfc shRNAs, but not WGEF shRNA, suppressed Dvl- and Wnt-3a-induced neurite retraction. p114-RhoGEF and Lfc shRNAs also inhibited Dvl- and Wnt-3a-induced RhoA activation, and p114-RhoGEF and Lfc proteins were capable of binding to Dvl and Daam1. Additionally, the Dvl-binding domains of p114-RhoGEF and Lfc inhibited Dvl-induced neurite retraction. Our results suggest that p114-RhoGEF and Lfc are critically involved in Wnt-3a- and Dvl-induced RhoA activation and neurite retraction in N1E-115 cells.
Figures
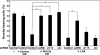


Similar articles
-
Dvl2-dependent activation of Daam1 and RhoA regulates Wnt5a-induced breast cancer cell migration.PLoS One. 2012;7(5):e37823. doi: 10.1371/journal.pone.0037823. Epub 2012 May 24. PLoS One. 2012. PMID: 22655072 Free PMC article.
-
Wnt-3a and Dvl induce neurite retraction by activating Rho-associated kinase.Mol Cell Biol. 2004 May;24(10):4487-501. doi: 10.1128/MCB.24.10.4487-4501.2004. Mol Cell Biol. 2004. PMID: 15121866 Free PMC article.
-
WGEF activates Rho in the Wnt-PCP pathway and controls convergent extension in Xenopus gastrulation.EMBO J. 2008 Feb 20;27(4):606-17. doi: 10.1038/emboj.2008.9. Epub 2008 Feb 7. EMBO J. 2008. PMID: 18256687 Free PMC article.
-
Dishevelled: The hub of Wnt signaling.Cell Signal. 2010 May;22(5):717-27. doi: 10.1016/j.cellsig.2009.11.021. Epub 2009 Dec 13. Cell Signal. 2010. PMID: 20006983 Review.
-
Rhogef17: A novel target for endothelial barrier function.Biomed Pharmacother. 2024 Jan;170:115983. doi: 10.1016/j.biopha.2023.115983. Epub 2023 Dec 21. Biomed Pharmacother. 2024. PMID: 38134633 Review.
Cited by
-
Passenger mutations and aberrant gene expression in congenic tissue plasminogen activator-deficient mouse strains.J Thromb Haemost. 2016 Aug;14(8):1618-28. doi: 10.1111/jth.13338. Epub 2016 Jun 20. J Thromb Haemost. 2016. PMID: 27079292 Free PMC article.
-
Homozygous ARHGEF2 mutation causes intellectual disability and midbrain-hindbrain malformation.PLoS Genet. 2017 Apr 28;13(4):e1006746. doi: 10.1371/journal.pgen.1006746. eCollection 2017 Apr. PLoS Genet. 2017. PMID: 28453519 Free PMC article.
-
PI3Kα isoform-dependent activation of RhoA regulates Wnt5a-induced osteosarcoma cell migration.Cancer Cell Int. 2017 Feb 14;17:27. doi: 10.1186/s12935-017-0396-8. eCollection 2017. Cancer Cell Int. 2017. PMID: 28289332 Free PMC article.
-
Go with the flow: GEF-H1 mediated shear stress mechanotransduction in neutrophils.Small GTPases. 2020 Jan;11(1):23-31. doi: 10.1080/21541248.2017.1332505. Epub 2017 Nov 30. Small GTPases. 2020. PMID: 29188751 Free PMC article. Review.
-
Dvl2-dependent activation of Daam1 and RhoA regulates Wnt5a-induced breast cancer cell migration.PLoS One. 2012;7(5):e37823. doi: 10.1371/journal.pone.0037823. Epub 2012 May 24. PLoS One. 2012. PMID: 22655072 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

