Human alveolar type II cells secrete and absorb liquid in response to local nucleotide signaling
- PMID: 20801871
- PMCID: PMC2966108
- DOI: 10.1074/jbc.M110.162933
Human alveolar type II cells secrete and absorb liquid in response to local nucleotide signaling
Abstract
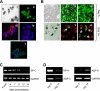
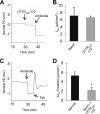
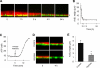
A balance sheet describing the integrated homeostasis of secretion, absorption, and surface movement of liquids on pulmonary surfaces has remained elusive. It remains unclear whether the alveolus exhibits an intra-alveolar ion/liquid transport physiology or whether it secretes ions/liquid that may communicate with airway surfaces. Studies employing isolated human alveolar type II (AT2) cells were utilized to investigate this question. Human AT2 cells exhibited both epithelial Na(+) channel-mediated Na(+) absorption and cystic fibrosis transmembrane conductance regulator-mediated Cl(-) secretion, both significantly regulated by extracellular nucleotides. In addition, we observed in normal AT2 cells an absence of cystic fibrosis transmembrane conductance regulator regulation of epithelial Na(+) channel activity and an absence of expression/activity of reported calcium-activated chloride channels (TMEM16A, Bestrophin-1, ClC2, and SLC26A9), both features strikingly different from normal airway epithelial cells. Measurements of alveolar surface liquid volume revealed that normal AT2 cells: 1) achieved an extracellular nucleotide concentration-dependent steady state alveolar surface liquid height of ∼4 μm in vitro; 2) absorbed liquid when the lumen was flooded; and 3) secreted liquid when treated with UTP or forskolin or subjected to cyclic compressive stresses mimicking tidal breathing. Collectively, our studies suggest that human AT2 cells in vitro have the capacity to absorb or secrete liquid in response to local alveolar conditions.
Figures

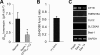

Similar articles
-
The role of Cl- in the regulation of ion and liquid transport in the intact alveolus during β-adrenergic stimulation.Exp Physiol. 2013 Feb;98(2):576-84. doi: 10.1113/expphysiol.2012.066159. Epub 2012 Aug 7. Exp Physiol. 2013. PMID: 22872661
-
UTP regulation of ion transport in alveolar epithelial cells involves distinct mechanisms.Am J Physiol Lung Cell Mol Physiol. 2009 Sep;297(3):L439-54. doi: 10.1152/ajplung.90268.2008. Epub 2009 Jun 19. Am J Physiol Lung Cell Mol Physiol. 2009. PMID: 19542245 Free PMC article.
-
Adrenergic regulation of ion transport across adult alveolar epithelial cells: effects on Cl- channel activation and transport function in cultures with an apical air interface.J Membr Biol. 2001 Jun 1;181(3):195-204. doi: 10.1007/s00232-001-0022-4. J Membr Biol. 2001. PMID: 11420606
-
Salt and water transport across the alveolar epithelium in the developing lung: correlations between function and recent molecular biology advances (Review).Int J Mol Med. 1998 Nov;2(5):515-31. doi: 10.3892/ijmm.2.5.515. Int J Mol Med. 1998. PMID: 9858647 Review.
-
Liquid movement across the surface epithelium of large airways.Respir Physiol Neurobiol. 2007 Dec 15;159(3):256-70. doi: 10.1016/j.resp.2007.06.005. Epub 2007 Jun 17. Respir Physiol Neurobiol. 2007. PMID: 17692578 Free PMC article. Review.
Cited by
-
Cellular Mechanisms of Lung Injury: Current Perspectives.Clin Chest Med. 2024 Dec;45(4):821-833. doi: 10.1016/j.ccm.2024.08.004. Epub 2024 Sep 20. Clin Chest Med. 2024. PMID: 39443000 Review.
-
Chloride secretion across adult alveolar epithelial cells contributes to cardiogenic edema.Proc Natl Acad Sci U S A. 2013 Jun 18;110(25):10055-6. doi: 10.1073/pnas.1307480110. Epub 2013 May 29. Proc Natl Acad Sci U S A. 2013. PMID: 23720313 Free PMC article. No abstract available.
-
Nadph oxidase regulates alveolar epithelial sodium channel activity and lung fluid balance in vivo via O⁻₂ signaling.Am J Physiol Lung Cell Mol Physiol. 2012 Feb 15;302(4):L410-9. doi: 10.1152/ajplung.00260.2011. Epub 2011 Dec 9. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22160304 Free PMC article.
-
Imaging and characterization of stretch-induced ATP release from alveolar A549 cells.J Physiol. 2013 Mar 1;591(5):1195-215. doi: 10.1113/jphysiol.2012.244145. Epub 2012 Dec 17. J Physiol. 2013. PMID: 23247110 Free PMC article.
-
Anoctamins.Pflugers Arch. 2011 Aug;462(2):195-208. doi: 10.1007/s00424-011-0975-9. Epub 2011 May 21. Pflugers Arch. 2011. PMID: 21607626 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

