Development and regeneration of sensory transduction in auditory hair cells requires functional interaction between cadherin-23 and protocadherin-15
- PMID: 20739546
- PMCID: PMC2949085
- DOI: 10.1523/JNEUROSCI.1949-10.2010
Development and regeneration of sensory transduction in auditory hair cells requires functional interaction between cadherin-23 and protocadherin-15
Abstract
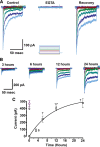
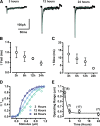
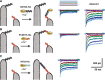
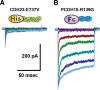
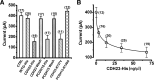
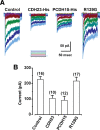
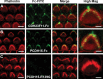
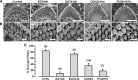
Tip links are extracellular filaments that connect pairs of hair cell stereocilia and convey tension to mechanosensitive channels. Recent evidence suggests that tip links are formed by calcium-dependent interactions between the N-terminal domains of cadherin-23 (CDH23) and protocadherin-15 (PCDH15). Mutations in either CDH23 or PCDH15 cause deafness in mice and humans, indicating the molecules are required for normal inner ear function. However, there is little physiological evidence to support a direct role for CDH23 and PCDH15 in hair cell mechanotransduction. To investigate the contributions of CDH23 and PCDH15 to mechanotransduction and tip-link formation, we examined outer hair cells of mouse cochleas during development and after chemical disruption of tip links. We found that tip links and mechanotransduction with all the qualitative properties of mature transduction recovered within 24 h after disruption. To probe tip-link formation, we measured transduction currents after extracellular application of recombinant CDH23 and PCDH15 fragments, which included putative interaction domains (EC1). Both fragments inhibited development and regeneration of transduction but did not disrupt transduction in mature cells. PCDH15 fragments that carried a mutation in EC1 that causes deafness in humans did not inhibit transduction development or regeneration. Immunolocalization revealed wild-type fragments bound near the tips of hair cell stereocilia. Scanning electron micrographs revealed that hair bundles exposed to fragments had a reduced number of linkages aligned along the morphological axis of sensitivity of the bundle. Together, the data provide direct evidence implicating CDH23 and PCDH15 proteins in the formation of tip links during development and regeneration of mechanotransduction.
Figures
Similar articles
-
Molecular remodeling of tip links underlies mechanosensory regeneration in auditory hair cells.PLoS Biol. 2013;11(6):e1001583. doi: 10.1371/journal.pbio.1001583. Epub 2013 Jun 11. PLoS Biol. 2013. PMID: 23776407 Free PMC article.
-
Tuning Inner-Ear Tip-Link Affinity Through Alternatively Spliced Variants of Protocadherin-15.Biochemistry. 2018 Mar 20;57(11):1702-1710. doi: 10.1021/acs.biochem.7b01075. Epub 2018 Mar 6. Biochemistry. 2018. PMID: 29443515 Free PMC article.
-
Mutations in protocadherin 15 and cadherin 23 affect tip links and mechanotransduction in mammalian sensory hair cells.PLoS One. 2011 Apr 21;6(4):e19183. doi: 10.1371/journal.pone.0019183. PLoS One. 2011. PMID: 21532990 Free PMC article.
-
Cadherins and mechanotransduction by hair cells.Curr Opin Cell Biol. 2008 Oct;20(5):557-66. doi: 10.1016/j.ceb.2008.06.004. Epub 2008 Jul 30. Curr Opin Cell Biol. 2008. PMID: 18619539 Free PMC article. Review.
-
The tip-link molecular complex of the auditory mechano-electrical transduction machinery.Hear Res. 2015 Dec;330(Pt A):10-7. doi: 10.1016/j.heares.2015.05.005. Epub 2015 Jun 3. Hear Res. 2015. PMID: 26049141 Review.
Cited by
-
The molecules that mediate sensory transduction in the mammalian inner ear.Curr Opin Neurobiol. 2015 Oct;34:165-71. doi: 10.1016/j.conb.2015.06.013. Epub 2015 Jul 25. Curr Opin Neurobiol. 2015. PMID: 26218316 Free PMC article. Review.
-
Underestimated sensitivity of mammalian cochlear hair cells due to splay between stereociliary columns.Biophys J. 2015 Jun 2;108(11):2633-47. doi: 10.1016/j.bpj.2015.04.028. Biophys J. 2015. PMID: 26039165 Free PMC article.
-
Distinct functions of TMC channels: a comparative overview.Cell Mol Life Sci. 2019 Nov;76(21):4221-4232. doi: 10.1007/s00018-019-03214-1. Epub 2019 Oct 4. Cell Mol Life Sci. 2019. PMID: 31584127 Free PMC article. Review.
-
Origin of inner ear hair cells: morphological and functional differentiation from ciliary cells into hair cells in zebrafish inner ear.J Neurosci. 2011 Mar 9;31(10):3784-94. doi: 10.1523/JNEUROSCI.5554-10.2011. J Neurosci. 2011. PMID: 21389233 Free PMC article.
-
Beyond Cell-Cell Adhesion: Sensational Cadherins for Hearing and Balance.Cold Spring Harb Perspect Biol. 2018 Sep 4;10(9):a029280. doi: 10.1101/cshperspect.a029280. Cold Spring Harb Perspect Biol. 2018. PMID: 28847902 Free PMC article. Review.
References
-
- Ahmed ZM, Riazuddin S, Ahmad J, Bernstein SL, Guo Y, Sabar MF, Sieving P, Riazuddin S, Griffith AJ, Friedman TB, Belyantseva IA, Wilcox ER. PCDH15 is expressed in the neurosensory epithelium of the eye and ear and mutant alleles are responsible for both USH1F and DFNB23. Hum Mol Genet. 2003;12:3215–3223. - PubMed
-
- Ahmed ZM, Goodyear R, Riazuddin S, Lagziel A, Legan PK, Behra M, Burgess SM, Lilley KS, Wilcox ER, Riazuddin S, Griffith AJ, Frolenkov GI, Belyantseva IA, Richardson GP, Friedman TB. The tip link antigen, a protein associated with the transduction complex of sensory hair cells, is protocadherin-15. J Neurosci. 2006;26:7022–7034. - PMC - PubMed
-
- Alagramam KN, Yuan H, Kuehn MH, Murcia CL, Wayne S, Srisailpathy CR, Lowry RB, Knaus R, Van Laer L, Bernier FP, Schwartz S, Lee C, Morton CC, Mullins RF, Ramesh A, Van Camp G, Hageman GS, Woychik RP, Smith RJ, Hagemen GS. Mutations in the novel protocadherin PCDH15 cause Usher syndrome type 1F. Hum Mol Genet. 2001;10:1709–1718. - PubMed
-
- Assad JA, Shepherd GM, Corey DP. Tip link integrity and mechanical transduction in vertebrate hair cells. Neuron. 1991;7:985–994. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
