Angiotensin II overcomes strain-dependent resistance of rapid CKD progression in a new remnant kidney mouse model
- PMID: 20736988
- PMCID: PMC3113489
- DOI: 10.1038/ki.2010.287
Angiotensin II overcomes strain-dependent resistance of rapid CKD progression in a new remnant kidney mouse model
Abstract
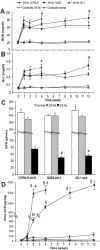
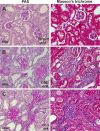
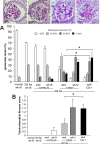
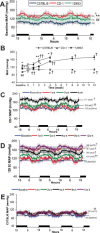
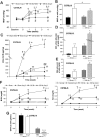
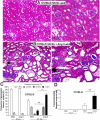
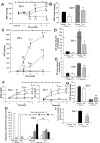
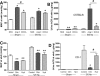
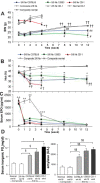
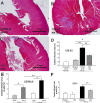
The remnant kidney model in C57BL/6 mice does not develop progressive chronic kidney disease (CKD). In this study we modified the model to mimic features of human CKD and to define accelerants of disease progression using three strains of mice. Following the procedure, there was a progressive increase in albuminuria, progressive loss in renal function, severe glomerulosclerosis and interstitial fibrosis, hypertension, cardiac fibrosis, and anemia by 4 weeks in CD-1 mice and by 12 weeks in 129S3 mice. In contrast, even after 16 weeks, the C57BL/6 mice with a remnant kidney had modestly increased albuminuria without increased blood pressure and without developing CKD or cardiac fibrosis. The baseline blood pressure, determined by radiotelemetry in conscious animals, correlated with CKD progression rates in each strain. Administering angiotensin II overcame the resistance of C57BL/6 mice to CKD following renal mass reduction, displaying high blood pressure and albuminuria, severe glomerulosclerosis, and loss of renal function by 4 weeks. Decreasing blood pressure with olmesartan, but not hydralazine, in CD-1 mice with a remnant kidney reduced CKD progression and cardiac fibrosis. C57BL/6 mice with a remnant kidney and DOCA-salt hypertension developed modest CKD. Each strain had similar degrees of interstitial fibrosis in three different normotensive models of renal fibrosis. Thus, reducing renal mass in CD-1 or 129S3 mice mimics many features of human CKD. Angiotensin II can convert the C57BL/6 strain from CKD resistant to susceptible in this disease model.
Figures
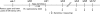
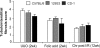
Similar articles
-
Renin inhibition ameliorates renal damage through prominent suppression of both angiotensin I and II in human renin angiotensinogen transgenic mice with high salt loading.Clin Exp Nephrol. 2014 Aug;18(4):593-9. doi: 10.1007/s10157-013-0893-6. Epub 2013 Oct 24. Clin Exp Nephrol. 2014. PMID: 24154707
-
Pharmacological inhibition of the NLRP3 inflammasome reduces blood pressure, renal damage, and dysfunction in salt-sensitive hypertension.Cardiovasc Res. 2019 Mar 15;115(4):776-787. doi: 10.1093/cvr/cvy252. Cardiovasc Res. 2019. PMID: 30357309 Free PMC article.
-
The N-type and L-type calcium channel blocker cilnidipine suppresses renal injury in Dahl rats fed a high-salt diet.Heart Vessels. 2010 Nov;25(6):549-55. doi: 10.1007/s00380-010-0005-4. Epub 2010 Oct 5. Heart Vessels. 2010. PMID: 20922532
-
The angiotensin-II (AT-II) receptor blocker olmesartan reduces renal damage in animal models of hypertension and diabetes.Proc West Pharmacol Soc. 2005;48:35-8. Proc West Pharmacol Soc. 2005. PMID: 16416656 Review.
-
Longitudinal changes in blood pressure are preceded by changes in albuminuria and accelerated by increasing dietary sodium intake.Exp Gerontol. 2023 Mar;173:112114. doi: 10.1016/j.exger.2023.112114. Epub 2023 Feb 6. Exp Gerontol. 2023. PMID: 36738979 Free PMC article. Review.
Cited by
-
Involvement of lysyl oxidase in the pathogenesis of arterial stiffness in chronic kidney disease.Am J Physiol Renal Physiol. 2023 Apr 1;324(4):F364-F373. doi: 10.1152/ajprenal.00239.2022. Epub 2023 Feb 24. Am J Physiol Renal Physiol. 2023. PMID: 36825626 Free PMC article.
-
A therapeutic target for CKD: activin A facilitates TGFβ1 profibrotic signaling.Cell Mol Biol Lett. 2023 Jan 30;28(1):10. doi: 10.1186/s11658-023-00424-1. Cell Mol Biol Lett. 2023. PMID: 36717814 Free PMC article.
-
Factor Xa inhibitor, edoxaban ameliorates renal injury after subtotal nephrectomy by reducing epithelial-mesenchymal transition and inflammatory response.Physiol Rep. 2022 Mar;10(5):e15218. doi: 10.14814/phy2.15218. Physiol Rep. 2022. PMID: 35262272 Free PMC article.
-
Animal Models of Kidney Disease: Challenges and Perspectives.Kidney360. 2023 Oct 1;4(10):1479-1493. doi: 10.34067/KID.0000000000000227. Kidney360. 2023. PMID: 37526653 Free PMC article. Review.
-
Ebselen reversed peripheral oxidative stress induced by a mouse model of sporadic Alzheimer's disease.Mol Biol Rep. 2020 Mar;47(3):2205-2215. doi: 10.1007/s11033-020-05326-5. Epub 2020 Feb 24. Mol Biol Rep. 2020. PMID: 32095983
References
-
- K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. - PubMed
-
- Patel SS, Kimmel PL, Singh A. New clinical practice guidelines for chronic kidney disease: a framework for K/DOQI. Semin Nephrol. 2002;22:449–458. - PubMed
-
- Hossain MP, Goyder EC, Rigby JE, et al. CKD and poverty: a growing global challenge. Am J Kidney Dis. 2009;53:166–174. - PubMed
-
- Coresh J, Astor BC, Greene T, et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. - PubMed
-
- Mitka M. Report notes increase in kidney disease. JAMA. 2008;300:2473–2474. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

