Adoptive transfer of PR1 cytotoxic T lymphocytes associated with reduced leukemia burden in a mouse acute myeloid leukemia xenograft model
- PMID: 20735170
- PMCID: PMC3365857
- DOI: 10.3109/14653249.2010.506506
Adoptive transfer of PR1 cytotoxic T lymphocytes associated with reduced leukemia burden in a mouse acute myeloid leukemia xenograft model
Abstract
Background aims: Tumor antigen-specific cytotoxic T lymphocytes (CTL) have been used in the treatment of human cancer, including leukemia. Several studies have established PR1 peptide, an HLA-A2.1-restricted peptide derived from proteinase 3 (P3), as a human leukemia-associated antigen. PR1-specific CTL elicited in vitro from healthy donors have been shown to lyse P3-expressing AML cells from patients. We investigated whether PR1-CTL can be adoptively transferred into NOD/SCID mice to eliminate human leukemia cells.
Methods: PR1-CTL were generated in bulk culture from peripheral blood mononuclear cells (PBMC) stimulated with autologous dendritic cells. Human acute myeloid leukemia (AML) patient samples were injected and engrafted in murine bone marrow at 2 weeks post-transfer.
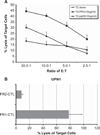
Results: Following adoptive transfer, bone marrow aspirate from mice that received AML alone had 72-88% blasts in a hypercellular marrow, whereas mice that received AML plus PR1-CTL co-infusion had normal hematopoietic elements and only 3-18% blasts in a hypocellular marrow. The PR1-CTL persisted in the bone marrow and liver and maintained a CD45RA⁻CD28+ effector phenotype.
Conclusions: We found that adoptive transfer of PR1-CTL generated in vitro is associated with reduced AML cells in NOD/SCID mice. PR1-CTL can migrate to the sites of disease and maintain their capacity to kill the AML cells. The surface phenotype of PR1-CTL was consistent with their trafficking pattern in both vascular and end-organ tissues.
Conflict of interest statement
Figures


Similar articles
-
A novel TCR-like CAR with specificity for PR1/HLA-A2 effectively targets myeloid leukemia in vitro when expressed in human adult peripheral blood and cord blood T cells.Cytotherapy. 2016 Aug;18(8):985-994. doi: 10.1016/j.jcyt.2016.05.001. Epub 2016 Jun 2. Cytotherapy. 2016. PMID: 27265873 Free PMC article.
-
PR1-specific cytotoxic T lymphocytes are relatively frequent in umbilical cord blood and can be effectively expanded to target myeloid leukemia.Cytotherapy. 2016 Aug;18(8):995-1001. doi: 10.1016/j.jcyt.2016.05.007. Cytotherapy. 2016. PMID: 27378343 Free PMC article.
-
Cytotoxic T lymphocytes specific for a nonpolymorphic proteinase 3 peptide preferentially inhibit chronic myeloid leukemia colony-forming units.Blood. 1997 Oct 1;90(7):2529-34. Blood. 1997. PMID: 9326217
-
Patient-individualized CD8⁺ cytolytic T-cell therapy effectively combats minimal residual leukemia in immunodeficient mice.Int J Cancer. 2016 Mar 1;138(5):1256-68. doi: 10.1002/ijc.29854. Epub 2015 Oct 5. Int J Cancer. 2016. PMID: 26376181
-
Antitumor cytotoxic T-lymphocyte response in human lung carcinoma: identification of a tumor-associated antigen.Immunol Rev. 2002 Oct;188:114-21. doi: 10.1034/j.1600-065x.2002.18810.x. Immunol Rev. 2002. PMID: 12445285 Review.
Cited by
-
Metabolic engineering and transcriptomic analysis of Saccharomyces cerevisiae producing p-coumaric acid from xylose.Microb Cell Fact. 2019 Nov 5;18(1):191. doi: 10.1186/s12934-019-1244-4. Microb Cell Fact. 2019. PMID: 31690329 Free PMC article.
-
Immunotherapy for acute myeloid leukemia: from allogeneic stem cell transplant to novel therapeutics.Leuk Lymphoma. 2019 Dec;60(14):3350-3362. doi: 10.1080/10428194.2019.1639167. Epub 2019 Jul 23. Leuk Lymphoma. 2019. PMID: 31335250 Free PMC article. Review.
-
Trial Watch: Adoptive TCR-Engineered T-Cell Immunotherapy for Acute Myeloid Leukemia.Cancers (Basel). 2021 Sep 8;13(18):4519. doi: 10.3390/cancers13184519. Cancers (Basel). 2021. PMID: 34572745 Free PMC article. Review.
-
Broad cross-presentation of the hematopoietically derived PR1 antigen on solid tumors leads to susceptibility to PR1-targeted immunotherapy.J Immunol. 2012 Dec 1;189(11):5476-84. doi: 10.4049/jimmunol.1201221. Epub 2012 Oct 26. J Immunol. 2012. PMID: 23105141 Free PMC article.
-
New approaches for the immunotherapy of acute myeloid leukemia.Discov Med. 2015 Apr;19(105):275-84. Discov Med. 2015. PMID: 25977190 Free PMC article. Review.
References
-
- Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. - PubMed
-
- Appelbaum FR. Haematopoietic cell transplantation as immunotherapy. Nature. 2001;411:385–389. - PubMed
-
- Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. - PubMed
-
- Goulmy E. Human minor histocompatibility antigens: new concepts for marrow transplantation and adoptive immunotherapy. Immunol Rev. 1997;157:125–140. - PubMed
-
- den Haan JM, Meadows LM, Wang W, Pool J, Blokland E, Bishop TL, et al. The minor histocompatibility antigen HA-1: a diallelic gene with a single amino acid polymorphism. Science. 1998;279:1054–1057. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

