Specific resistance to Pseudomonas aeruginosa infection in zebrafish is mediated by the cystic fibrosis transmembrane conductance regulator
- PMID: 20732993
- PMCID: PMC2976322
- DOI: 10.1128/IAI.00302-10
Specific resistance to Pseudomonas aeruginosa infection in zebrafish is mediated by the cystic fibrosis transmembrane conductance regulator
Abstract
Cystic fibrosis (CF) is a genetic disease caused by recessive mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene and is associated with prevalent and chronic Pseudomonas aeruginosa lung infections. Despite numerous studies that have sought to elucidate the role of CFTR in the innate immune response, the links between CFTR, innate immunity, and P. aeruginosa infection remain unclear. The present work highlights the zebrafish as a powerful model organism for human infectious disease, particularly infection by P. aeruginosa. Zebrafish embryos with reduced expression of the cftr gene (Cftr morphants) exhibited reduced respiratory burst response and directed neutrophil migration, supporting a connection between cftr and the innate immune response. Cftr morphants were infected with P. aeruginosa or other bacterial species that are commonly associated with infections in CF patients, including Burkholderia cenocepacia, Haemophilus influenzae, and Staphylococcus aureus. Intriguingly, the bacterial burden of P. aeruginosa was found to be significantly higher in zebrafish Cftr morphants than in controls, but this phenomenon was not observed with the other bacterial species. Bacterial burden in Cftr morphants infected with a P. aeruginosa ΔLasR mutant, a quorum sensing-deficient strain, was comparable to that in control fish, indicating that the regulation of virulence factors through LasR is required for enhancement of infection in the absence of Cftr. The zebrafish system provides a multitude of advantages for studying the pathogenesis of P. aeruginosa and for understanding the role that innate immune cells, such as neutrophils, play in the host response to acute bacterial infections commonly associated with cystic fibrosis.
Figures
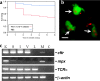
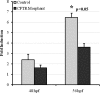

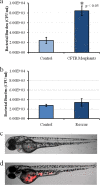
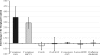
Similar articles
-
Quorum Sensing Down-Regulation Counteracts the Negative Impact of Pseudomonas aeruginosa on CFTR Channel Expression, Function and Rescue in Human Airway Epithelial Cells.Front Cell Infect Microbiol. 2017 Nov 10;7:470. doi: 10.3389/fcimb.2017.00470. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 29177135 Free PMC article.
-
Airway epithelial control of Pseudomonas aeruginosa infection in cystic fibrosis.Trends Mol Med. 2008 Mar;14(3):120-33. doi: 10.1016/j.molmed.2008.01.002. Epub 2008 Feb 11. Trends Mol Med. 2008. PMID: 18262467 Free PMC article. Review.
-
Harnessing Neutrophil Survival Mechanisms during Chronic Infection by Pseudomonas aeruginosa: Novel Therapeutic Targets to Dampen Inflammation in Cystic Fibrosis.Front Cell Infect Microbiol. 2017 Jun 30;7:243. doi: 10.3389/fcimb.2017.00243. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28713772 Free PMC article. Review.
-
Cystic fibrosis transmembrane conductance regulator and caveolin-1 regulate epithelial cell internalization of Pseudomonas aeruginosa.Am J Physiol Cell Physiol. 2009 Aug;297(2):C263-77. doi: 10.1152/ajpcell.00527.2008. Epub 2009 Apr 22. Am J Physiol Cell Physiol. 2009. PMID: 19386787 Free PMC article.
-
LasR Variant Cystic Fibrosis Isolates Reveal an Adaptable Quorum-Sensing Hierarchy in Pseudomonas aeruginosa.mBio. 2016 Oct 4;7(5):e01513-16. doi: 10.1128/mBio.01513-16. mBio. 2016. PMID: 27703072 Free PMC article.
Cited by
-
Intestinal Serum amyloid A suppresses systemic neutrophil activation and bactericidal activity in response to microbiota colonization.PLoS Pathog. 2019 Mar 7;15(3):e1007381. doi: 10.1371/journal.ppat.1007381. eCollection 2019 Mar. PLoS Pathog. 2019. PMID: 30845179 Free PMC article.
-
Testing multiple hypotheses through IMP weighted FDR based on a genetic functional network with application to a new zebrafish transcriptome study.BioData Min. 2015 Jun 17;8:17. doi: 10.1186/s13040-015-0050-8. eCollection 2015. BioData Min. 2015. PMID: 26097506 Free PMC article.
-
Deletion of cftr Leads to an Excessive Neutrophilic Response and Defective Tissue Repair in a Zebrafish Model of Sterile Inflammation.Front Immunol. 2020 Jul 31;11:1733. doi: 10.3389/fimmu.2020.01733. eCollection 2020. Front Immunol. 2020. PMID: 32849617 Free PMC article.
-
Dual Transcriptomics of Host-Pathogen Interaction of Cystic Fibrosis Isolate Pseudomonas aeruginosa PASS1 With Zebrafish.Front Cell Infect Microbiol. 2018 Nov 22;8:406. doi: 10.3389/fcimb.2018.00406. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30524971 Free PMC article.
-
mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish.Blood. 2011 Jan 27;117(4):e49-56. doi: 10.1182/blood-2010-10-314120. Epub 2010 Nov 17. Blood. 2011. PMID: 21084707 Free PMC article.
References
-
- Brannon, M. K., J. M. Davis, J. R. Mathias, C. J. Hall, J. C. Emerson, P. S. Crosier, A. Huttenlocher, L. Ramakrishnan, and S. M. Moskowitz. 2009. Pseudomonas aeruginosa type III secretion system interacts with phagocytes to modulate systemic infection of zebrafish embryos. Cell. Microbiol. 11:755-768. - PMC - PubMed
-
- Chen, J. M., C. Cutler, C. Jacques, G. Boeuf, E. Denamur, G. Lecointre, B. Mercier, G. Cramb, and C. Ferec. 2001. A combined analysis of the cystic fibrosis transmembrane conductance regulator: implications for structure and disease models. Mol. Biol. Evol. 18:1771-1788. - PubMed
-
- Chroneos, Z. C., S. E. Wert, J. L. Livingston, D. J. Hassett, and J. A. Whitsett. 2000. Role of cystic fibrosis transmembrane conductance regulator in pulmonary clearance of Pseudomonas aeruginosa in vivo. J. Immunol. 165:3941-3950. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

