The role of p53 in cell metabolism
- PMID: 20729871
- PMCID: PMC4002322
- DOI: 10.1038/aps.2010.151
The role of p53 in cell metabolism
Abstract
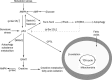
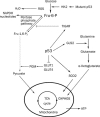
The p53 tumor suppressor gene has recently been shown to mediate metabolic changes in cells under physiological and pathological conditions. It has been revealed that p53 regulates energy metabolism, oxidative stress, and amino acid metabolism through balancing glycolysis and oxidative phosphorylation (OXPHOS) as well as the autophagy pathway. p53 is activated by metabolic stress through AMP-activated protein kinase (AMPK) and the mammalian target of rapamycin (mTOR) signaling pathways. p53 regulates OXPHOS through the transcriptional regulation of fructose-2,6-bisphosophatase, TP53-induced glycolysis regulator (TIGAR) and synthesis of cytochrome c oxidase (SCO2) subunit of complex IV of the electron transport chain. p53 also indirectly influences the energy metabolism through regulating glucose transporter (GLUT) expression, glutaminase 2 (GLS2) and fatty acid synthase (FAS). In addition, p53 regulates autophagy to provide cell metabolites for surviving through damage regulated autophagy modulator (DRAM1). Here we review the recent findings to elucidate the important role of p53 in cell metabolism.
Figures
Similar articles
-
Regulation of glucose metabolism by p53: emerging new roles for the tumor suppressor.Oncotarget. 2011 Dec;2(12):948-57. doi: 10.18632/oncotarget.389. Oncotarget. 2011. PMID: 22248668 Free PMC article. Review.
-
A systematic review of p53 regulation of oxidative stress in skeletal muscle.Redox Rep. 2018 Dec;23(1):100-117. doi: 10.1080/13510002.2017.1416773. Epub 2018 Jan 3. Redox Rep. 2018. PMID: 29298131 Free PMC article. Review.
-
Two p53-related metabolic regulators, TIGAR and SCO2, contribute to oroxylin A-mediated glucose metabolism in human hepatoma HepG2 cells.Int J Biochem Cell Biol. 2013 Jul;45(7):1468-78. doi: 10.1016/j.biocel.2013.04.015. Epub 2013 Apr 21. Int J Biochem Cell Biol. 2013. PMID: 23612020
-
SCO2 induces p53-mediated apoptosis by Thr845 phosphorylation of ASK-1 and dissociation of the ASK-1-Trx complex.Mol Cell Biol. 2013 Apr;33(7):1285-302. doi: 10.1128/MCB.06798-11. Epub 2013 Jan 14. Mol Cell Biol. 2013. PMID: 23319048 Free PMC article.
-
SCO2 Mediates Oxidative Stress-Induced Glycolysis to Oxidative Phosphorylation Switch in Hematopoietic Stem Cells.Stem Cells. 2016 Apr;34(4):960-71. doi: 10.1002/stem.2260. Epub 2015 Dec 31. Stem Cells. 2016. PMID: 26676373 Free PMC article.
Cited by
-
Interactions of SARS-CoV-2 with Human Target Cells-A Metabolic View.Int J Mol Sci. 2024 Sep 16;25(18):9977. doi: 10.3390/ijms25189977. Int J Mol Sci. 2024. PMID: 39337465 Free PMC article. Review.
-
Method for lipidomic analysis: p53 expression modulation of sulfatide, ganglioside, and phospholipid composition of U87 MG glioblastoma cells.Anal Chem. 2007 Nov 15;79(22):8423-30. doi: 10.1021/ac071413m. Epub 2007 Oct 12. Anal Chem. 2007. PMID: 17929901 Free PMC article.
-
Inhibition of p53-MDM2 binding reduces senescent cell abundance and improves the adaptive responses of skeletal muscle from aged mice.Geroscience. 2024 Apr;46(2):2153-2176. doi: 10.1007/s11357-023-00976-2. Epub 2023 Oct 24. Geroscience. 2024. PMID: 37872294 Free PMC article.
-
The genomic response of human granulosa cells (KGN) to melatonin and specific agonists/antagonists to the melatonin receptors.Sci Rep. 2022 Oct 20;12(1):17539. doi: 10.1038/s41598-022-21162-y. Sci Rep. 2022. PMID: 36266374 Free PMC article.
-
Development of cancer-initiating cells and immortalized cells with genomic instability.World J Stem Cells. 2015 Mar 26;7(2):483-9. doi: 10.4252/wjsc.v7.i2.483. World J Stem Cells. 2015. PMID: 25815132 Free PMC article. Review.
References
-
- Levine A. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–31. - PubMed
-
- Vogelstein B, Lane D, Levine A. Surfing the p53 network. Nature. 2000;408:307–10. - PubMed
-
- Hardie DG. The AMP-activated protein kinase pathway — new players upstream and downstream. J Cell Sci. 2004;117:5479–87. - PubMed
-
- Inoki K, Zhu T, Guan K. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

