A kinetochore-independent mechanism drives anaphase chromosome separation during acentrosomal meiosis
- PMID: 20729837
- PMCID: PMC3052858
- DOI: 10.1038/ncb2093
A kinetochore-independent mechanism drives anaphase chromosome separation during acentrosomal meiosis
Abstract
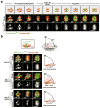
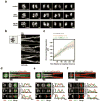
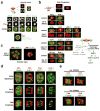
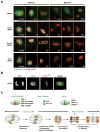
Although assembly of acentrosomal meiotic spindles has been extensively studied, little is known about the segregation of chromosomes on these spindles. Here, we show in Caenorhabditis elegans oocytes that the kinetochore protein, KNL-1, directs assembly of meiotic kinetochores that orient chromosomes. However, in contrast to mitosis, chromosome separation during meiotic anaphase is kinetochore-independent. Before anaphase, meiotic kinetochores and spindle poles disassemble along with the microtubules on the poleward side of chromosomes. During anaphase, microtubules then form between the separating chromosomes. Functional analysis implicated a set of proteins that localize to a ring-shaped domain between kinetochores during pre-anaphase spindle assembly and anaphase separation. These proteins are localized by the chromosomal passenger complex, which regulates the loss of meiotic chromosome cohesion. Thus, meiotic segregation in C. elegans is a two-stage process, where kinetochores orient chromosomes, but are then dispensable for their separation. We suggest that separation is controlled by a meiosis-specific chromosomal domain to coordinate cohesin removal and chromosome segregation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Comment in
-
Meiotic kinetochores get pushed aside by a CLS act.Nat Cell Biol. 2010 Sep;12(9):849-51. doi: 10.1038/ncb0910-849. Epub 2010 Aug 22. Nat Cell Biol. 2010. PMID: 20729839
Similar articles
-
Meiotic kinetochores get pushed aside by a CLS act.Nat Cell Biol. 2010 Sep;12(9):849-51. doi: 10.1038/ncb0910-849. Epub 2010 Aug 22. Nat Cell Biol. 2010. PMID: 20729839
-
Kinetochore-independent chromosome poleward movement during anaphase of meiosis II in mouse eggs.PLoS One. 2009;4(4):e5249. doi: 10.1371/journal.pone.0005249. Epub 2009 Apr 13. PLoS One. 2009. PMID: 19365562 Free PMC article.
-
The aurora B kinase AIR-2 regulates kinetochores during mitosis and is required for separation of homologous Chromosomes during meiosis.Curr Biol. 2002 May 14;12(10):798-812. doi: 10.1016/s0960-9822(02)00820-5. Curr Biol. 2002. PMID: 12015116
-
Chromosome dynamics: new light on Aurora B kinase function.Curr Biol. 2002 Jul 9;12(13):R458-60. doi: 10.1016/s0960-9822(02)00945-4. Curr Biol. 2002. PMID: 12121637 Review.
-
Mechanisms of kinesin-7 CENP-E in kinetochore-microtubule capture and chromosome alignment during cell division.Biol Cell. 2019 Jun;111(6):143-160. doi: 10.1111/boc.201800082. Epub 2019 Feb 26. Biol Cell. 2019. PMID: 30784092 Review.
Cited by
-
Maternal MEMI Promotes Female Meiosis II in Response to Fertilization in Caenorhabditis elegans.Genetics. 2016 Dec;204(4):1461-1477. doi: 10.1534/genetics.116.192997. Epub 2016 Oct 11. Genetics. 2016. PMID: 27729423 Free PMC article.
-
To Break or Not To Break: Sex Chromosome Hemizygosity During Meiosis in Caenorhabditis.Genetics. 2016 Nov;204(3):999-1013. doi: 10.1534/genetics.116.194308. Epub 2016 Sep 7. Genetics. 2016. PMID: 27605052 Free PMC article.
-
Male meiotic spindle features that efficiently segregate paired and lagging chromosomes.Elife. 2020 Mar 10;9:e50988. doi: 10.7554/eLife.50988. Elife. 2020. PMID: 32149606 Free PMC article.
-
A SUMO-Dependent Protein Network Regulates Chromosome Congression during Oocyte Meiosis.Mol Cell. 2017 Jan 5;65(1):66-77. doi: 10.1016/j.molcel.2016.11.001. Epub 2016 Dec 8. Mol Cell. 2017. PMID: 27939944 Free PMC article.
-
Anaphase A: Disassembling Microtubules Move Chromosomes toward Spindle Poles.Biology (Basel). 2017 Feb 17;6(1):15. doi: 10.3390/biology6010015. Biology (Basel). 2017. PMID: 28218660 Free PMC article. Review.
References
-
- Dumont J, Brunet S. Meiotic Spindle Assembly and Chromosome Segregation in Oocytes. In: Verlhac MH, Villeneuve AM, editors. Oogenesis: The Universal Process. Wiley-Blackwell; 2010. pp. 269–290.
-
- Kaitna S, Pasierbek P, Jantsch M, Loidl J, Glotzer M. The aurora B kinase AIR-2 regulates kinetochores during mitosis and is required for separation of homologous Chromosomes during meiosis. Curr Biol. 2002;12:798–812. - PubMed
-
- Maddox PS, Oegema K, Desai A, Cheeseman IM. “Holo”er than thou: chromosome segregation and kinetochore function in C. elegans. Chromosome Res. 2004;12:641–653. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

