The postsynaptic adenomatous polyposis coli (APC) multiprotein complex is required for localizing neuroligin and neurexin to neuronal nicotinic synapses in vivo
- PMID: 20720115
- PMCID: PMC2945243
- DOI: 10.1523/JNEUROSCI.0983-10.2010
The postsynaptic adenomatous polyposis coli (APC) multiprotein complex is required for localizing neuroligin and neurexin to neuronal nicotinic synapses in vivo
Abstract
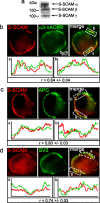
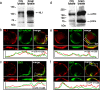
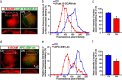
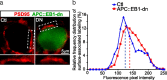
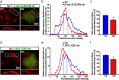
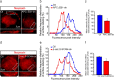
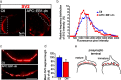
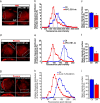
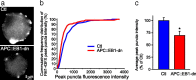
Synaptic efficacy requires that presynaptic and postsynaptic specializations align precisely and mature coordinately. The underlying mechanisms are poorly understood, however. We propose that adenomatous polyposis coli protein (APC) is a key coordinator of presynaptic and postsynaptic maturation. APC organizes a multiprotein complex that directs nicotinic acetylcholine receptor (nAChR) localization at postsynaptic sites in avian ciliary ganglion neurons in vivo. We hypothesize that the APC complex also provides retrograde signals that direct presynaptic active zones to develop in register with postsynaptic nAChR clusters. In our model, the APC complex provides retrograde signals via postsynaptic neuroligin that interacts extracellularly with presynaptic neurexin. S-SCAM (synaptic cell adhesion molecule) and PSD-93 (postsynaptic density-93) are scaffold proteins that bind to neuroligin. We identify S-SCAM as a novel component of neuronal nicotinic synapses. We show that S-SCAM, PSD-93, neuroligin and neurexin are enriched at alpha3*-nAChR synapses. PSD-93 and S-SCAM bind to APC and its binding partner beta-catenin, respectively. Blockade of selected APC and beta-catenin interactions, in vivo, leads to decreased postsynaptic accumulation of S-SCAM, but not PSD-93. Importantly, neuroligin synaptic clusters are also decreased. On the presynaptic side, there are decreases in neurexin and active zone proteins. Further, presynaptic terminals are less mature structurally and functionally. We define a novel neural role for APC by showing that the postsynaptic APC multiprotein complex is required for anchoring neuroligin and neurexin at neuronal synapses in vivo. APC human gene mutations correlate with autism spectrum disorders, providing strong support for the importance of the association, demonstrated here, between APC, neuroligin and neurexin.
Figures

Similar articles
-
Neuronal nicotinic synapse assembly requires the adenomatous polyposis coli tumor suppressor protein.J Neurosci. 2004 Jul 28;24(30):6776-84. doi: 10.1523/JNEUROSCI.1826-04.2004. J Neurosci. 2004. PMID: 15282282 Free PMC article.
-
Adenomatous polyposis coli plays a key role, in vivo, in coordinating assembly of the neuronal nicotinic postsynaptic complex.Mol Cell Neurosci. 2008 Jun;38(2):138-52. doi: 10.1016/j.mcn.2008.02.006. Epub 2008 Mar 4. Mol Cell Neurosci. 2008. PMID: 18407517 Free PMC article.
-
Capabilities of neurexins in the chick ciliary ganglion.Dev Neurobiol. 2008 Feb 15;68(3):409-19. doi: 10.1002/dneu.20598. Dev Neurobiol. 2008. PMID: 18161851
-
Postsynaptic scaffolds for nicotinic receptors on neurons.Acta Pharmacol Sin. 2009 Jun;30(6):694-701. doi: 10.1038/aps.2009.52. Epub 2009 May 11. Acta Pharmacol Sin. 2009. PMID: 19434056 Free PMC article. Review.
-
Intracellular protein complexes involved in synapse assembly in presynaptic neurons.Adv Protein Chem Struct Biol. 2019;116:347-373. doi: 10.1016/bs.apcsb.2018.11.008. Epub 2018 Dec 20. Adv Protein Chem Struct Biol. 2019. PMID: 31036296 Review.
Cited by
-
MAGUKs, synaptic development, and synaptic plasticity.Neuroscientist. 2011 Oct;17(5):493-512. doi: 10.1177/1073858410386384. Epub 2011 Apr 15. Neuroscientist. 2011. PMID: 21498811 Free PMC article. Review.
-
Ubiquitous neurocognitive dysfunction in familial adenomatous polyposis: proof-of-concept of the role of APC protein in neurocognitive function.Hered Cancer Clin Pract. 2020 Feb 24;18:4. doi: 10.1186/s13053-020-0135-3. eCollection 2020. Hered Cancer Clin Pract. 2020. PMID: 32123549 Free PMC article.
-
Social Stimulus Causes Aberrant Activation of the Medial Prefrontal Cortex in a Mouse Model With Autism-Like Behaviors.Front Synaptic Neurosci. 2018 Oct 12;10:35. doi: 10.3389/fnsyn.2018.00035. eCollection 2018. Front Synaptic Neurosci. 2018. PMID: 30369876 Free PMC article.
-
Epigenomic Changes after Acupuncture Treatment in Patients Suffering from Burnout.Complement Med Res. 2022;29(2):109-119. doi: 10.1159/000521347. Epub 2021 Dec 7. Complement Med Res. 2022. PMID: 34875647 Free PMC article.
-
Cell surface localization of α3β4 nicotinic acetylcholine receptors is regulated by N-cadherin homotypic binding and actomyosin contractility.PLoS One. 2013 Apr 23;8(4):e62435. doi: 10.1371/journal.pone.0062435. Print 2013. PLoS One. 2013. PMID: 23626818 Free PMC article.
References
-
- Boucard AA, Chubykin AA, Comoletti D, Taylor P, Südhof TC. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron. 2005;48:229–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
