Analysis of the signaling pathways regulating Src-dependent remodeling of the actin cytoskeleton
- PMID: 20719402
- PMCID: PMC3005982
- DOI: 10.1016/j.ejcb.2010.07.006
Analysis of the signaling pathways regulating Src-dependent remodeling of the actin cytoskeleton
Abstract
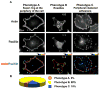
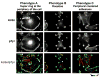
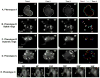
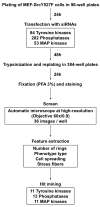
Cell adhesion to the extracellular matrix is mediated by adhesion receptors, mainly integrins, which upon interaction with the extracellular matrix, bind to the actin cytoskeleton via their cytoplasmic domains. This association is mediated by a variety of scaffold and signaling proteins, which control the mechanical and signaling activities of the adhesion site. Upon transformation of fibroblasts with active forms of Src (e.g., v-Src), focal adhesions are disrupted, and transformed into dot-like contacts known as podosomes, and consisting of a central actin core surrounded by an adhesion ring. To clarify the mechanism underlying Src-dependent modulation of the adhesive phenotype, and its influence on podosome organization, we screened for the effect of siRNA-mediated knockdown of tyrosine kinases, MAP kinases and phosphatases on the reorganization of the adhesion-cytoskeleton complex, induced by a constitutively active Src mutant (SrcY527F). In this screen, we discovered several genes that are involved in Src-induced remodeling of the actin cytoskeleton. We further showed that knockdown of Src in osteoclasts abolishes the formation of the podosome-based rings and impairs cell spreading, without inducing stress fiber development. Our work points to several genes that are involved in this process, and sheds new light on the molecular plasticity of integrin adhesions.
Copyright © 2010 Elsevier GmbH. All rights reserved.
Figures



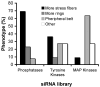
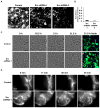
Similar articles
-
Dynamin forms a Src kinase-sensitive complex with Cbl and regulates podosomes and osteoclast activity.Mol Biol Cell. 2005 Jul;16(7):3301-13. doi: 10.1091/mbc.e04-12-1117. Epub 2005 May 4. Mol Biol Cell. 2005. PMID: 15872089 Free PMC article.
-
Protein tyrosine phosphatase epsilon regulates integrin-mediated podosome stability in osteoclasts by activating Src.Mol Biol Cell. 2009 Oct;20(20):4324-34. doi: 10.1091/mbc.e08-11-1158. Epub 2009 Aug 19. Mol Biol Cell. 2009. PMID: 19692574 Free PMC article.
-
SRC-mediated phosphorylation of focal adhesion kinase couples actin and adhesion dynamics to survival signaling.Mol Cell Biol. 2004 Sep;24(18):8113-33. doi: 10.1128/MCB.24.18.8113-8133.2004. Mol Cell Biol. 2004. PMID: 15340073 Free PMC article.
-
The Actin Network Interfacing Diverse Integrin-Mediated Adhesions.Biomolecules. 2023 Feb 4;13(2):294. doi: 10.3390/biom13020294. Biomolecules. 2023. PMID: 36830665 Free PMC article. Review.
-
The interplay between Src and integrins in normal and tumor biology.Oncogene. 2004 Oct 18;23(48):7928-46. doi: 10.1038/sj.onc.1208080. Oncogene. 2004. PMID: 15489911 Review.
Cited by
-
Proteomic Markers for Mechanobiological Properties of Metastatic Cancer Cells.Int J Mol Sci. 2023 Mar 1;24(5):4773. doi: 10.3390/ijms24054773. Int J Mol Sci. 2023. PMID: 36902201 Free PMC article. Review.
-
Dasatinib suppresses invasion and induces apoptosis in nasopharyngeal carcinoma.Int J Clin Exp Pathol. 2015 Jul 1;8(7):7818-24. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26339346 Free PMC article.
-
Mechanical Cues Affect Migration and Invasion of Cells From Three Different Directions.Front Cell Dev Biol. 2020 Sep 17;8:583226. doi: 10.3389/fcell.2020.583226. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33043017 Free PMC article. Review.
-
Regulation of osteoclasts by membrane-derived lipid mediators.Cell Mol Life Sci. 2013 Sep;70(18):3341-53. doi: 10.1007/s00018-012-1238-4. Epub 2013 Jan 8. Cell Mol Life Sci. 2013. PMID: 23296124 Free PMC article. Review.
-
VEGF/VEGFR2 signaling regulates hippocampal axon branching during development.Elife. 2019 Dec 23;8:e49818. doi: 10.7554/eLife.49818. Elife. 2019. PMID: 31868583 Free PMC article.
References
-
- Berrier AL, Yamada KM. Cell-matrix adhesion. J Cell Physiol. 2007;213:565–573. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

