Oxidative status of muscle is determined by p107 regulation of PGC-1alpha
- PMID: 20713602
- PMCID: PMC2928004
- DOI: 10.1083/jcb.201005076
Oxidative status of muscle is determined by p107 regulation of PGC-1alpha
Abstract
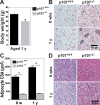
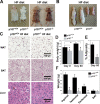
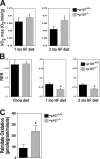
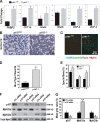
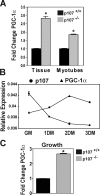
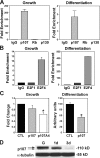
Mice lacking p107 exhibit a white adipose deficiency yet do not manifest the metabolic changes typical for lipodystrophy, and instead exhibit low levels of serum triglycerides and a normal liver phenotype. When fed a high fat diet, p107-null mice still did not accumulate fat in the liver, and display markedly elevated energy expenditures together with an increased energy preference for lipids. Skeletal muscle was therefore examined, as this is normally the major tissue involved in whole body lipid metabolism. Notably, p107-deficient muscle express increased levels of peroxisome proliferator-activated receptor gamma co-activator-1alpha (PGC-1alpha) and contained increased numbers of the pro-oxidative type I and type IIa myofibers. Chromatin immunoprecipitation revealed binding of p107 and E2F4 to the PGC-1alpha proximal promoter, and this binding repressed promoter activity in transient transcription assays. Ectopic expression of p107 in muscle tissue in vivo results in a pronounced 20% decrease in the numbers of oxidative type IIa myofibers. Lastly, isolated p107-deficient muscle tissue display a threefold increase in lipid metabolism. Therefore, p107 determines the oxidative state of multiple tissues involved in whole body fat metabolism, including skeletal muscle.
Figures

Similar articles
-
Rb and p107 regulate preadipocyte differentiation into white versus brown fat through repression of PGC-1alpha.Cell Metab. 2005 Nov;2(5):283-95. doi: 10.1016/j.cmet.2005.10.002. Cell Metab. 2005. PMID: 16271529
-
Coordinated balancing of muscle oxidative metabolism through PGC-1α increases metabolic flexibility and preserves insulin sensitivity.Biochem Biophys Res Commun. 2011 Apr 29;408(1):180-5. doi: 10.1016/j.bbrc.2011.04.012. Epub 2011 Apr 8. Biochem Biophys Res Commun. 2011. PMID: 21501593
-
Peroxisome proliferator-activated receptor {gamma} coactivator 1{alpha} (PGC-1{alpha}) promotes skeletal muscle lipid refueling in vivo by activating de novo lipogenesis and the pentose phosphate pathway.J Biol Chem. 2010 Oct 22;285(43):32793-32800. doi: 10.1074/jbc.M110.145995. Epub 2010 Aug 17. J Biol Chem. 2010. PMID: 20716531 Free PMC article.
-
High-fat diet-induced reduction of peroxisome proliferator-activated receptor-γ coactivator-1α messenger RNA levels and oxidative capacity in the soleus muscle of rats with metabolic syndrome.Nutr Res. 2012 Feb;32(2):144-51. doi: 10.1016/j.nutres.2011.12.015. Nutr Res. 2012. PMID: 22348463
-
PGC-1alpha-induced improvements in skeletal muscle metabolism and insulin sensitivity.Appl Physiol Nutr Metab. 2009 Jun;34(3):307-14. doi: 10.1139/H09-008. Appl Physiol Nutr Metab. 2009. PMID: 19448691 Review.
Cited by
-
Running forward: new frontiers in endurance exercise biology.Circulation. 2014 Feb 18;129(7):798-810. doi: 10.1161/CIRCULATIONAHA.113.001590. Circulation. 2014. PMID: 24550551 Free PMC article. Review. No abstract available.
-
p107-Dependent recruitment of SWI/SNF to the alkaline phosphatase promoter during osteoblast differentiation.Bone. 2014 Dec;69:47-54. doi: 10.1016/j.bone.2014.08.009. Epub 2014 Aug 23. Bone. 2014. PMID: 25182511 Free PMC article.
-
Transcriptional control of stem cell fate by E2Fs and pocket proteins.Front Genet. 2015 Apr 28;6:161. doi: 10.3389/fgene.2015.00161. eCollection 2015. Front Genet. 2015. PMID: 25972892 Free PMC article. Review.
-
Identification of eQTLs associated with lipid metabolism in Longissimus dorsi muscle of pigs with different genetic backgrounds.Sci Rep. 2020 Jun 17;10(1):9845. doi: 10.1038/s41598-020-67015-4. Sci Rep. 2020. PMID: 32555447 Free PMC article.
-
Coregulator-mediated control of skeletal muscle plasticity - A mini-review.Biochimie. 2017 May;136:49-54. doi: 10.1016/j.biochi.2016.12.011. Epub 2017 Jan 3. Biochimie. 2017. PMID: 28057584 Free PMC article. Review.
References
-
- Baldi A., Esposito V., De Luca A., Fu Y., Meoli I., Giordano G.G., Caputi M., Baldi F., Giordano A. 1997. Differential expression of Rb2/p130 and p107 in normal human tissues and in primary lung cancer. Clin. Cancer Res. 3:1691–1697 - PubMed
-
- Cameron-Smith D., Burke L.M., Angus D.J., Tunstall R.J., Cox G.R., Bonen A., Hawley J.A., Hargreaves M. 2003. A short-term, high-fat diet up-regulates lipid metabolism and gene expression in human skeletal muscle. Am. J. Clin. Nutr. 77:313–318 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

