Timing of cortical interneuron migration is influenced by the cortical hem
- PMID: 20713502
- PMCID: PMC3059882
- DOI: 10.1093/cercor/bhq142
Timing of cortical interneuron migration is influenced by the cortical hem
Abstract
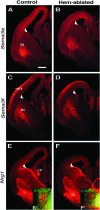
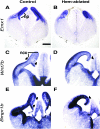
Cerebral cortical γ-aminobutyric acid (GABA)ergic interneurons originate from the basal forebrain and migrate into the cortex in 2 phases. First, interneurons cross the boundary between the developing striatum and the cortex to migrate tangentially through the cortical primordium. Second, interneurons migrate radially to their correct neocortical layer position. A previous study demonstrated that mice in which the cortical hem was genetically ablated displayed a massive reduction of Cajal-Retzius (C-R) cells in the neocortical marginal zone (MZ), thereby losing C-R cell-generated reelin in the MZ. Surprisingly, pyramidal cell migration and subsequent layering were almost normal. In contrast, we find that the timing of migration of cortical GABAergic interneurons is abnormal in hem-ablated mice. Migrating interneurons both advance precociously along their tangential path and switch prematurely from tangential to radial migration to invade the cortical plate (CP). We propose that the cortical hem is responsible for establishing cues that control the timing of interneuron migration. In particular, we suggest that loss of a repellant signal from the medial neocortex, which is greatly decreased in size in hem-ablated mice, allows the early advance of interneurons and that reduction of another secreted molecule from C-R cells, the chemokine SDF-1/CXCL12, permits early radial migration into the CP.
Figures

Similar articles
-
Multidirectional and multizonal tangential migration of GABAergic interneurons in the developing cerebral cortex.Development. 2006 Jun;133(11):2167-76. doi: 10.1242/dev.02382. Epub 2006 May 3. Development. 2006. PMID: 16672340
-
Postnatal shifts of interneuron position in the neocortex of normal and reeler mice: evidence for inward radial migration.Neuroscience. 2004;124(3):605-18. doi: 10.1016/j.neuroscience.2003.11.033. Neuroscience. 2004. PMID: 14980731
-
Connexin 43 mediates the tangential to radial migratory switch in ventrally derived cortical interneurons.J Neurosci. 2010 May 19;30(20):7072-7. doi: 10.1523/JNEUROSCI.5728-09.2010. J Neurosci. 2010. PMID: 20484649 Free PMC article.
-
Control of tangential/non-radial migration of neurons in the developing cerebral cortex.Neurochem Int. 2007 Jul-Sep;51(2-4):121-31. doi: 10.1016/j.neuint.2007.05.006. Epub 2007 May 21. Neurochem Int. 2007. PMID: 17588709 Review.
-
Molecules and mechanisms involved in the generation and migration of cortical interneurons.ASN Neuro. 2010 Mar 31;2(2):e00031. doi: 10.1042/AN20090053. ASN Neuro. 2010. PMID: 20360946 Free PMC article. Review.
Cited by
-
Decision making during interneuron migration in the developing cerebral cortex.Trends Cell Biol. 2014 Jun;24(6):342-51. doi: 10.1016/j.tcb.2013.12.001. Epub 2014 Jan 2. Trends Cell Biol. 2014. PMID: 24388877 Free PMC article. Review.
-
Functional synergy between cholecystokinin receptors CCKAR and CCKBR in mammalian brain development.PLoS One. 2015 Apr 15;10(4):e0124295. doi: 10.1371/journal.pone.0124295. eCollection 2015. PLoS One. 2015. PMID: 25875176 Free PMC article.
-
Aberrant survival of hippocampal Cajal-Retzius cells leads to memory deficits, gamma rhythmopathies and susceptibility to seizures in adult mice.Nat Commun. 2023 Mar 18;14(1):1531. doi: 10.1038/s41467-023-37249-7. Nat Commun. 2023. PMID: 36934089 Free PMC article.
-
Expression and functional analysis of the Wnt/beta-catenin induced mir-135a-2 locus in embryonic forebrain development.Neural Dev. 2016 Apr 5;11:9. doi: 10.1186/s13064-016-0065-y. Neural Dev. 2016. PMID: 27048518 Free PMC article.
-
Glycine neurotransmission: Its role in development.Front Neurosci. 2022 Sep 16;16:947563. doi: 10.3389/fnins.2022.947563. eCollection 2022. Front Neurosci. 2022. PMID: 36188468 Free PMC article. Review.
References
-
- Anderson S, Eisenstat D, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. - PubMed
-
- Anderson S, Mione M, Yun K, Rubenstein JL. Differential origins of neocortical projection and local circuit neurons: role of Dlx genes in neocortical interneuronogenesis. Cereb Cortex. 1999;9:646–654. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

