Mediation of T-cell activation by actin meshworks
- PMID: 20702599
- PMCID: PMC2926748
- DOI: 10.1101/cshperspect.a002444
Mediation of T-cell activation by actin meshworks
Abstract
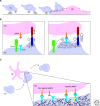
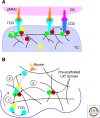
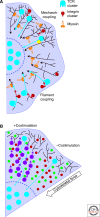
Although the actin cytoskeleton and T-cell receptor (TCR) signaling complexes are seemingly distinct molecular structures, they are tightly integrated in T cells. The signaling pathways initiated by TCRs binding to peptide MHC complexes are extensively influenced by the actin cytoskeletal activities of the motile phase before TCR signaling, the signalosome scaffolding function of the cytoskeleton, and the translocation of signaling clusters that precedes the termination of signaling at these complexes. As these three successive phases constitute essentially all the steps consequent to immune synapse formation, it has become clear that the substantial physical forces and signaling interactions generated by the actin cytoskeleton dominate the signaling life cycle of TCR signalosomes. We discuss the contributions of the actin cytoskeleton to TCR signaling phases and model some remaining questions about how specific cytoskeletal factors regulate TCR signaling outcomes.
Figures
Similar articles
-
Regulation of sustained actin dynamics by the TCR and costimulation as a mechanism of receptor localization.J Immunol. 2003 Sep 1;171(5):2287-95. doi: 10.4049/jimmunol.171.5.2287. J Immunol. 2003. PMID: 12928373
-
Regulation of T-cell receptor signaling by the actin cytoskeleton and poroelastic cytoplasm.Immunol Rev. 2013 Nov;256(1):148-59. doi: 10.1111/imr.12120. Immunol Rev. 2013. PMID: 24117819 Free PMC article. Review.
-
Changes in actin dynamics at the T-cell/APC interface: implications for T-cell anergy?Immunol Rev. 2002 Nov;189:98-110. doi: 10.1034/j.1600-065x.2002.18909.x. Immunol Rev. 2002. PMID: 12445268 Review.
-
Multiple actin networks coordinate mechanotransduction at the immunological synapse.J Cell Biol. 2020 Feb 3;219(2):e201911058. doi: 10.1083/jcb.201911058. J Cell Biol. 2020. PMID: 31977034 Free PMC article.
-
TCR, LFA-1, and CD28 play unique and complementary roles in signaling T cell cytoskeletal reorganization.J Immunol. 1999 Feb 1;162(3):1367-75. J Immunol. 1999. PMID: 9973391
Cited by
-
T cell activation requires force generation.J Cell Biol. 2016 Jun 6;213(5):535-42. doi: 10.1083/jcb.201511053. Epub 2016 May 30. J Cell Biol. 2016. PMID: 27241914 Free PMC article.
-
T cell antigen receptor activation and actin cytoskeleton remodeling.Biochim Biophys Acta. 2014 Feb;1838(2):546-56. doi: 10.1016/j.bbamem.2013.05.004. Epub 2013 May 14. Biochim Biophys Acta. 2014. PMID: 23680625 Free PMC article. Review.
-
The adaptor protein LAT serves as an integration node for signaling pathways that drive T cell activation.Wiley Interdiscip Rev Syst Biol Med. 2013 Jan-Feb;5(1):101-10. doi: 10.1002/wsbm.1194. Epub 2012 Nov 13. Wiley Interdiscip Rev Syst Biol Med. 2013. PMID: 23150273 Free PMC article. Review.
-
Regulation of CD8 T-cell signaling, metabolism, and cytotoxic activity by extracellular lysophosphatidic acid.Immunol Rev. 2023 Aug;317(1):203-222. doi: 10.1111/imr.13208. Epub 2023 Apr 25. Immunol Rev. 2023. PMID: 37096808 Free PMC article. Review.
-
Actin Dynamics at the T Cell Synapse as Revealed by Immune-Related Actinopathies.Front Cell Dev Biol. 2021 Jun 24;9:665519. doi: 10.3389/fcell.2021.665519. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34249918 Free PMC article. Review.
References
-
- Barda-Saad M, Braiman A, Titerence R, Bunnell SC, Barr VA, Samelson LE 2005. Dynamic molecular interactions linking the T cell antigen receptor to the actin cytoskeleton. Nat Immunol 6: 80–89 - PubMed
-
- Boniface JJ, Rabinowitz JD, Wülfing C, Hampl J, Reich Z, Altman JD, Kantor RM, Beeson C, McConnell HM, Davis MM 1998. Initiation of signal transduction through the T cell receptor requires the multivalent engagement of peptide/MHC ligands. Immunity 9: 459–466 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
