Proteomics of dense core secretory vesicles reveal distinct protein categories for secretion of neuroeffectors for cell-cell communication
- PMID: 20695487
- PMCID: PMC2996463
- DOI: 10.1021/pr1003104
Proteomics of dense core secretory vesicles reveal distinct protein categories for secretion of neuroeffectors for cell-cell communication
Abstract
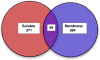
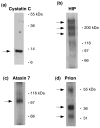
Regulated secretion of neurotransmitters and neurohumoral factors from dense core secretory vesicles provides essential neuroeffectors for cell-cell communication in the nervous and endocrine systems. This study provides comprehensive proteomic characterization of the categories of proteins in chromaffin dense core secretory vesicles that participate in cell-cell communication from the adrenal medulla. Proteomic studies were conducted by nano-HPLC Chip MS/MS tandem mass spectrometry. Results demonstrate that these secretory vesicles contain proteins of distinct functional categories consisting of neuropeptides and neurohumoral factors, protease systems, neurotransmitter enzymes and transporters, receptors, enzymes for biochemical processes, reduction/oxidation regulation, ATPases, protein folding, lipid biochemistry, signal transduction, exocytosis, calcium regulation, as well as structural and cell adhesion proteins. The secretory vesicle proteomic data identified 371 proteins in the soluble fraction and 384 membrane proteins, for a total of 686 distinct secretory vesicle proteins. Notably, these proteomic analyses illustrate the presence of several neurological disease-related proteins in these secretory vesicles, including huntingtin interacting protein, cystatin C, ataxin 7, and prion protein. Overall, these findings demonstrate that multiple protein categories participate in dense core secretory vesicles for production, storage, and secretion of bioactive neuroeffectors for cell-cell communication in health and disease.
Figures


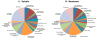


Similar articles
-
Proteomics of neuroendocrine secretory vesicles reveal distinct functional systems for biosynthesis and exocytosis of peptide hormones and neurotransmitters.J Proteome Res. 2007 May;6(5):1652-65. doi: 10.1021/pr060503p. Epub 2007 Apr 5. J Proteome Res. 2007. PMID: 17408250
-
Mass spectrometry-based neuropeptidomics of secretory vesicles from human adrenal medullary pheochromocytoma reveals novel peptide products of prohormone processing.J Proteome Res. 2010 Oct 1;9(10):5065-75. doi: 10.1021/pr100358b. J Proteome Res. 2010. PMID: 20704348 Free PMC article.
-
Electron microscopic evidence for multiple types of secretory vesicles in bovine chromaffin cells.Gen Comp Endocrinol. 2001 Mar;121(3):261-77. doi: 10.1006/gcen.2000.7592. Gen Comp Endocrinol. 2001. PMID: 11254368
-
The adrenal chromaffin granule: a model for large dense core vesicles of endocrine and nervous tissue.J Anat. 1993 Oct;183 ( Pt 2)(Pt 2):237-52. J Anat. 1993. PMID: 8300414 Free PMC article. Review.
-
Morphological docking of secretory vesicles.Histochem Cell Biol. 2010 Aug;134(2):103-13. doi: 10.1007/s00418-010-0719-5. Epub 2010 Jun 26. Histochem Cell Biol. 2010. PMID: 20577884 Free PMC article. Review.
Cited by
-
Basement Membranes, Brittlestar Tendons, and Their Mechanical Adaptability.Biology (Basel). 2024 May 24;13(6):375. doi: 10.3390/biology13060375. Biology (Basel). 2024. PMID: 38927255 Free PMC article. Review.
-
Beta-amyloid peptides undergo regulated co-secretion with neuropeptide and catecholamine neurotransmitters.Peptides. 2013 Aug;46:126-35. doi: 10.1016/j.peptides.2013.04.020. Epub 2013 Jun 6. Peptides. 2013. PMID: 23747840 Free PMC article.
-
Resource allocation in mammalian systems.Biotechnol Adv. 2024 Mar-Apr;71:108305. doi: 10.1016/j.biotechadv.2023.108305. Epub 2024 Jan 11. Biotechnol Adv. 2024. PMID: 38215956 Review.
-
Cysteine Cathepsins in the secretory vesicle produce active peptides: Cathepsin L generates peptide neurotransmitters and cathepsin B produces beta-amyloid of Alzheimer's disease.Biochim Biophys Acta. 2012 Jan;1824(1):89-104. doi: 10.1016/j.bbapap.2011.08.015. Epub 2011 Sep 8. Biochim Biophys Acta. 2012. PMID: 21925292 Free PMC article. Review.
-
In vivo characterization of the maturation steps of a pigment dispersing factor neuropeptide precursor in the Drosophila circadian pacemaker neurons.Genetics. 2023 Aug 31;225(1):iyad118. doi: 10.1093/genetics/iyad118. Genetics. 2023. PMID: 37364299 Free PMC article.
References
-
- Gainer H, Russell JT, Loh YP. The enzymology and intracellular organization of peptide precursor processing: the secretory vesicle hypothesis. Neuroendocrinology. 1985;40:171–84. - PubMed
-
- Kim T, Gondré-Lewis MC, Arnaoutova I, Loh YP. Dense-core secretory granule biogenesis. Physiology. 2006;21:124–33. - PubMed
-
- Scalettar BA. How neurosecretory vesicles release their cargo. Neuroscientist. 2006;12:164–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH077305-05/MH/NIMH NIH HHS/United States
- R01EY014061/EY/NEI NIH HHS/United States
- R01 DA004271/DA/NIDA NIH HHS/United States
- R01 MH077305/MH/NIMH NIH HHS/United States
- R01NS24553/NS/NINDS NIH HHS/United States
- R01MH077305/MH/NIMH NIH HHS/United States
- R01 NS024553/NS/NINDS NIH HHS/United States
- K01 DA023065-04/DA/NIDA NIH HHS/United States
- K01 DA023065/DA/NIDA NIH HHS/United States
- R01 DA004271-23/DA/NIDA NIH HHS/United States
- P01HL58120/HL/NHLBI NIH HHS/United States
- R01DA04271/DA/NIDA NIH HHS/United States
- P01 HL058120/HL/NHLBI NIH HHS/United States
- P01 HL058120-100003/HL/NHLBI NIH HHS/United States
- P01 HL058120-109004/HL/NHLBI NIH HHS/United States
- R01 NS024553-19/NS/NINDS NIH HHS/United States
- K01DA023065/DA/NIDA NIH HHS/United States
- R01 EY014061-08/EY/NEI NIH HHS/United States
- R01 EY014061/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources

