Physiological roles of voltage-gated proton channels in leukocytes
- PMID: 20693294
- PMCID: PMC3010135
- DOI: 10.1113/jphysiol.2010.194225
Physiological roles of voltage-gated proton channels in leukocytes
Abstract
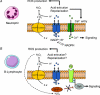
Voltage-gated proton channels are designed to extrude large quantities of cytosolic acid in response to depolarising voltages. The discovery of the Hvcn1 gene and the generation of mice lacking the channel molecule have confirmed several postulated functions of proton channels in leukocytes. In neutrophils and macrophages, proton channels are required for high-level production of superoxide anions by the phagocytic NADPH oxidase, a bactericidal enzyme essential for host defence against infections. In B lymphocytes, proton channels are required for low-level production of superoxide that boosts the production of antibodies. Proton channels sustain the activity of immune cells in several ways. By extruding excess cytosolic acid, proton channels prevent deleterious acidification of the cytosol and at the same time deliver protons required for chemical conversion of the superoxide secreted by membrane oxidases. By moving positive charges across membranes, proton channels limit the depolarisation of the plasma membrane, promoting the electrogenic activity of NADPH oxidases and the entry of calcium ions into cells. Acid extrusion by proton channels is not restricted to leukocytes but also mediates the intracellular alkalinisation required for the activation of spermatozoids. Proton channels are therefore multitalented channels that control male fertility as well as our innate and adaptive immunity.
Figures
Similar articles
-
Diversity of voltage gated proton channels.Front Biosci. 2003 Sep 1;8:s1266-79. doi: 10.2741/1191. Front Biosci. 2003. PMID: 12957841 Review.
-
Regulation of immune responses by proton channels.Immunology. 2014 Oct;143(2):131-7. doi: 10.1111/imm.12326. Immunology. 2014. PMID: 24890927 Free PMC article. Review.
-
Do Hv1 proton channels regulate the ionic and redox homeostasis of phagosomes?Mol Cell Endocrinol. 2012 Apr 28;353(1-2):82-7. doi: 10.1016/j.mce.2011.10.005. Epub 2011 Oct 26. Mol Cell Endocrinol. 2012. PMID: 22056415 Review.
-
Voltage-gated proton channels maintain pH in human neutrophils during phagocytosis.Proc Natl Acad Sci U S A. 2009 Oct 20;106(42):18022-7. doi: 10.1073/pnas.0905565106. Epub 2009 Oct 5. Proc Natl Acad Sci U S A. 2009. PMID: 19805063 Free PMC article.
-
Identification of Thr29 as a critical phosphorylation site that activates the human proton channel Hvcn1 in leukocytes.J Biol Chem. 2010 Feb 19;285(8):5117-21. doi: 10.1074/jbc.C109.082727. Epub 2009 Dec 26. J Biol Chem. 2010. PMID: 20037153 Free PMC article.
Cited by
-
Detection of superoxide anion and hydrogen peroxide production by cellular NADPH oxidases.Biochim Biophys Acta. 2014 Feb;1840(2):757-67. doi: 10.1016/j.bbagen.2013.04.040. Epub 2013 May 7. Biochim Biophys Acta. 2014. PMID: 23660153 Free PMC article. Review.
-
Construction and validation of a homology model of the human voltage-gated proton channel hHV1.J Gen Physiol. 2013 Apr;141(4):445-65. doi: 10.1085/jgp.201210856. J Gen Physiol. 2013. PMID: 23530137 Free PMC article.
-
Role of human Hv1 channels in sperm capacitation and white blood cell respiratory burst established by a designed peptide inhibitor.Proc Natl Acad Sci U S A. 2018 Dec 11;115(50):E11847-E11856. doi: 10.1073/pnas.1816189115. Epub 2018 Nov 26. Proc Natl Acad Sci U S A. 2018. PMID: 30478045 Free PMC article.
-
The redox-sensitive cation channel TRPM2 modulates phagocyte ROS production and inflammation.Nat Immunol. 2011 Nov 20;13(1):29-34. doi: 10.1038/ni.2171. Nat Immunol. 2011. PMID: 22101731 Free PMC article.
-
Proton production, regulation and pathophysiological roles in the mammalian brain.Neurosci Bull. 2012 Feb;28(1):1-13. doi: 10.1007/s12264-012-1068-2. Neurosci Bull. 2012. PMID: 22233885 Free PMC article. Review.
References
-
- Capasso M, Bhamrah MK, Henley T, Boyd RS, Langlais C, Cain K, Dinsdale D, Pulford K, Khan M, Musset B, Cherny VV, Morgan D, Gascoyne RD, Vigorito E, DeCoursey TE, MacLennan IC, Dyer MJ. HVCN1 modulates BCR signal strength via regulation of BCR-dependent generation of reactive oxygen species. Nat Immunol. 2010;11:265–272. - PMC - PubMed
-
- Decoursey TE. Voltage-gated proton channels and other proton transfer pathways. Physiol Rev. 2003;83:475–579. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

