Psychological stress-induced, IDO1-dependent tryptophan catabolism: implications on immunosuppression in mice and humans
- PMID: 20689575
- PMCID: PMC2911374
- DOI: 10.1371/journal.pone.0011825
Psychological stress-induced, IDO1-dependent tryptophan catabolism: implications on immunosuppression in mice and humans
Abstract
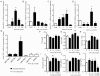
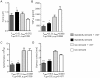
It is increasingly recognized that psychological stress influences inflammatory responses and mood. Here, we investigated whether psychological stress (combined acoustic and restraint stress) activates the tryptophan (Trp) catabolizing enzyme indoleamine 2,3-dioxygenase 1(IDO1) and thereby alters the immune homeostasis and behavior in mice. We measured IDO1 mRNA expression and plasma levels of Trp catabolites after a single 2-h stress session and in repeatedly stressed (4.5-days stress, 2-h twice a day) naïve BALB/c mice. A role of cytokines in acute stress-induced IDO1 activation was studied after IFNgamma and TNFalpha blockade and in IDO1(-/-) mice. RU486 and 1-Methyl-L-tryptophan (1-MT) were used to study role of glucocorticoids and IDO1 on Trp depletion in altering the immune and behavioral response in repeatedly stressed animals. Clinical relevance was addressed by analyzing IDO1 activity in patients expecting abdominal surgery. Acute stress increased the IDO1 mRNA expression in brain, lung, spleen and Peyer's patches (max. 14.1+/-4.9-fold in brain 6-h after stress) and resulted in a transient depletion of Trp (-25.2+/-6.6%) and serotonin (-27.3+/-4.6%) from the plasma measured 6-h after stress while kynurenine levels increased 6-h later (11.2+/-9.3%). IDO1 mRNA up-regulation was blocked by anti-TNFalpha and anti-IFNgamma treatment. Continuous IDO1 blockade by 1-MT but not RU486 treatment normalized the anti-bacterial defense and attenuated increased IL-10 inducibility in splenocytes after repeated stress as it reduced the loss of body weight and behavioral alterations. Moreover, kynurenic acid which remained increased in 1-MT treated repeatedly stressed mice was identified to reduce the TNFalpha inducibility of splenocytes in vitro and in vivo. Thus, psychological stress stimulates cytokine-driven IDO1 activation and Trp depletion which seems to have a central role for developing stress-induced immunosuppression and behavioral alteration. Since patients showed Trp catabolism already prior to surgery, IDO is also a possible target enzyme for humans modulating immune homeostasis and mood.
Conflict of interest statement
Figures
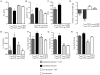
Similar articles
-
The Immunomodulator 1-Methyltryptophan Drives Tryptophan Catabolism Toward the Kynurenic Acid Branch.Front Immunol. 2020 Feb 28;11:313. doi: 10.3389/fimmu.2020.00313. eCollection 2020. Front Immunol. 2020. PMID: 32180772 Free PMC article.
-
The immune effects of TRYCATs (tryptophan catabolites along the IDO pathway): relevance for depression - and other conditions characterized by tryptophan depletion induced by inflammation.Neuro Endocrinol Lett. 2007 Dec;28(6):826-31. Neuro Endocrinol Lett. 2007. PMID: 18063923
-
Up-regulation of indoleamine 2,3-dioxygenase 1 (IDO1) expression and catalytic activity is associated with immunosuppression and poor prognosis in penile squamous cell carcinoma patients.Cancer Commun (Lond). 2020 Jan;40(1):3-15. doi: 10.1002/cac2.12001. Epub 2020 Mar 3. Cancer Commun (Lond). 2020. PMID: 32125093 Free PMC article.
-
The new '5-HT' hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression.Prog Neuropsychopharmacol Biol Psychiatry. 2011 Apr 29;35(3):702-21. doi: 10.1016/j.pnpbp.2010.12.017. Epub 2010 Dec 23. Prog Neuropsychopharmacol Biol Psychiatry. 2011. PMID: 21185346 Review.
-
Role of indoleamine 2,3-dioxygenase 1 (IDO1) and kynurenine pathway in the regulation of the aging process.Ageing Res Rev. 2022 Mar;75:101573. doi: 10.1016/j.arr.2022.101573. Epub 2022 Jan 24. Ageing Res Rev. 2022. PMID: 35085834 Review.
Cited by
-
Indolamine 2,3-dioxygenase regulation and neuropsychiatric symptoms.Psychoneuroendocrinology. 2013 Sep;38(9):1829-30. doi: 10.1016/j.psyneuen.2013.05.020. Epub 2013 Jun 27. Psychoneuroendocrinology. 2013. PMID: 23810316 Free PMC article. No abstract available.
-
Indoleamine-2,3-Dioxygenase as a Perioperative Marker of the Immune System.Front Physiol. 2021 Nov 8;12:766511. doi: 10.3389/fphys.2021.766511. eCollection 2021. Front Physiol. 2021. PMID: 34819875 Free PMC article. Review.
-
Prenatal kynurenine treatment in rats causes schizophrenia-like broad monitoring deficits in adulthood.Psychopharmacology (Berl). 2018 Mar;235(3):651-661. doi: 10.1007/s00213-017-4780-9. Epub 2017 Nov 11. Psychopharmacology (Berl). 2018. PMID: 29128872 Free PMC article.
-
Impact of Microbial Metabolites on Microbiota-Gut-Brain Axis in Inflammatory Bowel Disease.Int J Mol Sci. 2021 Feb 5;22(4):1623. doi: 10.3390/ijms22041623. Int J Mol Sci. 2021. PMID: 33562721 Free PMC article. Review.
-
Analysis of Metabolites in Gout: A Systematic Review and Meta-Analysis.Nutrients. 2023 Jul 14;15(14):3143. doi: 10.3390/nu15143143. Nutrients. 2023. PMID: 37513561 Free PMC article. Review.
References
-
- WHO publication. 2010. Mental health, depression. http://www.who.int/mental_health/management/depression/definition/en/: online publication.
-
- Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67:259–284. - PubMed
-
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. - PubMed
-
- Schmidt MV, Sterlemann V, Muller MB. Chronic stress and individual vulnerability. Ann N Y Acad Sci. 2008;1148:174–183. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

