Mononucleosis and antigen-driven T cell responses have different requirements for interleukin-2 signaling in murine gammaherpesvirus infection
- PMID: 20686022
- PMCID: PMC2950560
- DOI: 10.1128/JVI.00856-10
Mononucleosis and antigen-driven T cell responses have different requirements for interleukin-2 signaling in murine gammaherpesvirus infection
Abstract
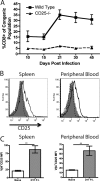
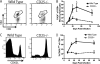
Interleukin-2 (IL-2) has been implicated as being necessary for the optimal formation of primary CD8(+) T cell responses against various pathogens. Here we have examined the role that IL-2 signaling plays in several aspects of a CD8(+) T cell response against murine gammaherpesvirus 68 (MHV-68). Exposure to MHV-68 causes a persistent infection, along with infectious mononucleosis, providing a model for studying these processes in mice. Our study indicates that CD25 is necessary for optimal expansion of the antigen-specific CD8(+) T cell response but not for the long-term memory response. Contrastingly, IL-2 signaling through CD25 is absolutely required for CD8(+) T cell mononucleosis.
Figures
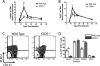
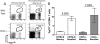
Similar articles
-
Type I interferon signaling enhances CD8+ T cell effector function and differentiation during murine gammaherpesvirus 68 infection.J Virol. 2014 Dec;88(24):14040-9. doi: 10.1128/JVI.02360-14. Epub 2014 Sep 24. J Virol. 2014. PMID: 25253356 Free PMC article.
-
A mouse model for infectious mononucleosis.Immunol Res. 2002;25(3):201-17. doi: 10.1385/IR:25:3:201. Immunol Res. 2002. PMID: 12018460 Review.
-
Functional heterogeneity in the CD4+ T cell response to murine γ-herpesvirus 68.J Immunol. 2015 Mar 15;194(6):2746-56. doi: 10.4049/jimmunol.1401928. Epub 2015 Feb 6. J Immunol. 2015. PMID: 25662997 Free PMC article.
-
Antigen expression during murine gamma-herpesvirus infection.Immunobiology. 2001 Dec;204(5):649-58. doi: 10.1078/0171-2985-00104. Immunobiology. 2001. PMID: 11846230 Review.
-
Murine γ-Herpesvirus 68 Induces Severe Lung Inflammation in IL-27-Deficient Mice with Liver Dysfunction Preventable by Oral Neomycin.J Immunol. 2018 Apr 15;200(8):2703-2713. doi: 10.4049/jimmunol.1700412. Epub 2018 Mar 2. J Immunol. 2018. PMID: 29500240
Cited by
-
Lytic Replication and Reactivation from B Cells Is Not Required for Establishing or Maintaining Gammaherpesvirus Latency In Vivo.J Virol. 2022 Jun 22;96(12):e0069022. doi: 10.1128/jvi.00690-22. Epub 2022 Jun 1. J Virol. 2022. PMID: 35647668 Free PMC article.
References
-
- Bachmann, M. F., P. Wolint, S. Walton, K. Schwarz, and A. Oxenius. 2007. Differential role of IL-2R signaling for CD8+ T cell responses in acute and chronic viral infections. Eur. J. Immunol. 37:1502-1512. - PubMed
-
- Belz, G. T., P. G. Stevenson, M. R. Castrucci, J. D. Altman, and P. C. Doherty. 2000. Postexposure vaccination massively increases the prevalence of gamma-herpesvirus-specific CD8+ T cells but confers minimal survival advantage on CD4-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 97:2725-2730. - PMC - PubMed
-
- Coppola, M. A., E. Flano, P. Nguyen, C. L. Hardy, R. D. Cardin, N. Shastri, D. L. Woodland, and M. A. Blackman. 1999. Apparent MHC-independent stimulation of CD8+ T cells in vivo during latent murine gammaherpesvirus infection. J. Immunol. 163:1481-1489. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

