Increased catecholamine secretion contributes to hypertension in TRPM4-deficient mice
- PMID: 20679729
- PMCID: PMC2929713
- DOI: 10.1172/JCI41348
Increased catecholamine secretion contributes to hypertension in TRPM4-deficient mice
Abstract
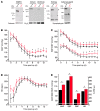
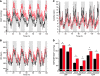
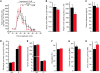
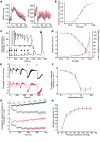
Hypertension is an underlying risk factor for cardiovascular disease. Despite this, its pathogenesis remains unknown in most cases. Recently, the transient receptor potential (TRP) channel family was associated with the development of several cardiovascular diseases linked to hypertension. The melastatin TRP channels TRPM4 and TRPM5 have distinct properties within the TRP channel family: they form nonselective cation channels activated by intracellular calcium ions. Here we report the identification of TRPM4 proteins in endothelial cells, heart, kidney, and chromaffin cells from the adrenal gland, suggesting that they have a role in the cardiovascular system. Consistent with this hypothesis, Trpm4 gene deletion in mice altered long-term regulation of blood pressure toward hypertensive levels. No changes in locomotor activity, renin-angiotensin system function, electrolyte and fluid balance, vascular contractility, and cardiac contractility under basal conditions were observed. By contrast, inhibition of ganglionic transmission with either hexamethonium or prazosin abolished the difference in blood pressure between Trpm4-/- and wild-type mice. Strikingly, plasma epinephrine concentration as well as urinary excretion of catecholamine metabolites were substantially elevated in Trpm4-/- mice. In freshly isolated chromaffin cells, lack of TRPM4 was shown to cause markedly more acetylcholine-induced exocytotic release events, while neither cytosolic calcium concentration, size, nor density of vesicles were different. We therefore conclude that TRPM4 proteins limit catecholamine release from chromaffin cells and that this contributes to increased sympathetic tone and hypertension.
Figures
Similar articles
-
The Ca(2+)-activated cation channel TRPM4 is a negative regulator of angiotensin II-induced cardiac hypertrophy.Basic Res Cardiol. 2015;110(4):43. doi: 10.1007/s00395-015-0501-x. Epub 2015 Jun 5. Basic Res Cardiol. 2015. PMID: 26043922 Free PMC article.
-
Adenylyl cyclase-mediated effects contribute to increased Isoprenaline-induced cardiac contractility in TRPM4-deficient mice.J Mol Cell Cardiol. 2014 Sep;74:307-17. doi: 10.1016/j.yjmcc.2014.06.007. Epub 2014 Jun 24. J Mol Cell Cardiol. 2014. PMID: 24972051
-
Genetic background influences expression and function of the cation channel TRPM4 in the mouse heart.Basic Res Cardiol. 2020 Nov 17;115(6):70. doi: 10.1007/s00395-020-00831-x. Basic Res Cardiol. 2020. PMID: 33205255 Free PMC article.
-
TRPM4 channels in the cardiovascular system: physiology, pathophysiology, and pharmacology.Biochem Pharmacol. 2012 Oct 1;84(7):873-81. doi: 10.1016/j.bcp.2012.06.021. Epub 2012 Jun 27. Biochem Pharmacol. 2012. PMID: 22750058 Review.
-
TRPM4 channels in the cardiovascular system.Curr Opin Pharmacol. 2014 Apr;15:68-73. doi: 10.1016/j.coph.2013.12.003. Epub 2013 Dec 25. Curr Opin Pharmacol. 2014. PMID: 24721656 Review.
Cited by
-
The Ca(2+)-activated cation channel TRPM4 is a negative regulator of angiotensin II-induced cardiac hypertrophy.Basic Res Cardiol. 2015;110(4):43. doi: 10.1007/s00395-015-0501-x. Epub 2015 Jun 5. Basic Res Cardiol. 2015. PMID: 26043922 Free PMC article.
-
Ion channel-kinase TRPM7 is required for maintaining cardiac automaticity.Proc Natl Acad Sci U S A. 2013 Aug 6;110(32):E3037-46. doi: 10.1073/pnas.1311865110. Epub 2013 Jul 22. Proc Natl Acad Sci U S A. 2013. PMID: 23878236 Free PMC article.
-
Research trends in transient receptor potential vanilloid in cardiovascular disease: Bibliometric analysis and visualization.Front Cardiovasc Med. 2023 Feb 22;10:1071198. doi: 10.3389/fcvm.2023.1071198. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 36910533 Free PMC article.
-
Disorders of blood pressure regulation-role of catecholamine biosynthesis, release, and metabolism.Curr Hypertens Rep. 2012 Feb;14(1):38-45. doi: 10.1007/s11906-011-0239-2. Curr Hypertens Rep. 2012. PMID: 22068338 Review.
-
Mechanosensitivity in Pulmonary Circulation: Pathophysiological Relevance of Stretch-Activated Channels in Pulmonary Hypertension.Biomolecules. 2021 Sep 21;11(9):1389. doi: 10.3390/biom11091389. Biomolecules. 2021. PMID: 34572602 Free PMC article. Review.
References
-
- Cowley AW., Jr The genetic dissection of essential hypertension. Nat Rev Genet. 2006;7(11):829–840. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

