Adenovirus 5 E1A enhances histone deacetylase inhibitors-induced apoptosis through Egr-1-mediated Bim upregulation
- PMID: 20676141
- PMCID: PMC2955174
- DOI: 10.1038/onc.2010.295
Adenovirus 5 E1A enhances histone deacetylase inhibitors-induced apoptosis through Egr-1-mediated Bim upregulation
Abstract
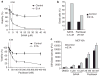
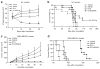
Histone deacetylase inhibitors (HDACi) are potent anti-cancer agents for variety of cancer types. Suberoylanilide hydroxamic acid (SAHA) has been approved as a drug to treat cutaneous T cell lymphoma, and the combination of HDACi and other agents have been actively tested in many clinical trials. Adenovirus 5 early region 1A (E1A) has been shown to exhibit high tumor suppressor activity, and gene therapy using E1A has been tested in clinical trials. Here, we showed that proapoptotic activity of HDACi was robustly enhanced by E1A in multiple cancer cells, but not in normal cells. Moreover, we showed that combination of E1A gene therapy and SAHA showed high therapeutic efficacy with low toxicity in vivo ovarian and breast xenograft models. SAHA downregulated Bcl-XL and upregulated proapoptotic BH3-only protein Bim, whose expression was further enhanced by E1A in cancer cells. These alterations of Bcl-2 family proteins were critical for apoptosis induced by the combination in cancer cells. SAHA enhanced acetylation of histone H3 in Bim promoter region, while E1A upregulated Egr-1, which was directly involved in Bim transactivation. Together, our results provide not only a novel insight into the mechanisms underlying anti-tumor activity of E1A, but also a rationale for the combined HDACi and E1A gene therapy in future clinical trials.
Conflict of interest statement
The corresponding author, Dr Mien-Chie Hung, is an inventor on patents covering E1A as a therapeutic agent filed by the University of Texas MD Anderson Cancer. The remaining authors declare no conflict of interest.
Figures





Similar articles
-
c-Myc overexpression sensitizes Bim-mediated Bax activation for apoptosis induced by histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) through regulating Bcl-2/Bcl-xL expression.Int J Biochem Cell Biol. 2007;39(5):1016-25. doi: 10.1016/j.biocel.2007.01.024. Epub 2007 Feb 2. Int J Biochem Cell Biol. 2007. PMID: 17331788
-
Vorinostat-induced apoptosis in mantle cell lymphoma is mediated by acetylation of proapoptotic BH3-only gene promoters.Clin Cancer Res. 2011 Jun 15;17(12):3956-68. doi: 10.1158/1078-0432.CCR-10-3412. Epub 2011 Jun 7. Clin Cancer Res. 2011. PMID: 21652541
-
Bim upregulation by histone deacetylase inhibitors mediates interactions with the Bcl-2 antagonist ABT-737: evidence for distinct roles for Bcl-2, Bcl-xL, and Mcl-1.Mol Cell Biol. 2009 Dec;29(23):6149-69. doi: 10.1128/MCB.01481-08. Epub 2009 Oct 5. Mol Cell Biol. 2009. PMID: 19805519 Free PMC article.
-
Mechanisms of Histone Deacetylase Inhibitor-Regulated Gene Expression in Cancer Cells.Antioxid Redox Signal. 2015 Jul 1;23(1):66-84. doi: 10.1089/ars.2014.5863. Epub 2014 Mar 27. Antioxid Redox Signal. 2015. PMID: 24512308 Free PMC article. Review.
-
Vorinostat (SAHA) and Breast Cancer: An Overview.Cancers (Basel). 2021 Sep 19;13(18):4700. doi: 10.3390/cancers13184700. Cancers (Basel). 2021. PMID: 34572928 Free PMC article. Review.
Cited by
-
Migfilin sensitizes cisplatin-induced apoptosis in human glioma cells in vitro.Acta Pharmacol Sin. 2012 Oct;33(10):1301-10. doi: 10.1038/aps.2012.123. Epub 2012 Sep 17. Acta Pharmacol Sin. 2012. PMID: 22983390 Free PMC article.
-
Ras Participates in the Regulation of the Stability of Adenoviral Protein E1A via MAP-kinase ERK.Acta Naturae. 2022 Apr-Jun;14(2):78-84. doi: 10.32607/actanaturae.11675. Acta Naturae. 2022. PMID: 35923563 Free PMC article.
-
Histone deacetylase inhibitor trichostatin A sensitises cisplatin-resistant ovarian cancer cells to oncolytic adenovirus.Oncotarget. 2018 May 29;9(41):26328-26341. doi: 10.18632/oncotarget.25242. eCollection 2018 May 29. Oncotarget. 2018. PMID: 29899862 Free PMC article.
-
The potential role of YAP in Axl-mediated resistance to EGFR tyrosine kinase inhibitors.Am J Cancer Res. 2019 Dec 1;9(12):2719-2729. eCollection 2019. Am J Cancer Res. 2019. PMID: 31911857 Free PMC article.
-
Overexpression of Adenoviral E1A Sensitizes E1A+Ras-Transformed Cells to the Action of Histone Deacetylase Inhibitors.Acta Naturae. 2018 Oct-Dec;10(4):70-78. Acta Naturae. 2018. PMID: 30713764 Free PMC article.
References
-
- Baek SJ, Wilson LC, Hsi LC, Eling TE. Troglitazone, a peroxisome proliferator-activated receptor gamma (PPAR gamma) ligand, selectively induces the early growth response-1 gene independently of PPAR gamma. A novel mechanism for its anti-tumorigenic activity. J Biol Chem. 2003;278:5845–5853. - PubMed
-
- Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. - PubMed
-
- Bouillet P, Zhang LC, Huang DC, Webb GC, Bottema CD, Shore P, et al. Gene structure alternative splicing, and chromosomal localization of pro-apoptotic Bcl-2 relative Bim. Mamm Genome. 2001;12:163–168. - PubMed
-
- Brader KR, Wolf JK, Hung MC, Yu D, Crispens MA, van Golen KL, et al. Adenovirus E1A expression enhances the sensitivity of an ovarian cancer cell line to multiple cytotoxic agents through an apoptotic mechanism. Clin Cancer Res. 1997;3:2017–2024. - PubMed
-
- Burgess A, Ruefli A, Beamish H, Warrener R, Saunders N, Johnstone R, et al. Histone deacetylase inhibitors specifically kill nonproliferating tumour cells. Oncogene. 2004;23:6693–6701. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

