Analysis of adenovirus trans-complementation-mediated gene expression controlled by melanoma-specific TETP promoter in vitro
- PMID: 20670430
- PMCID: PMC2920257
- DOI: 10.1186/1743-422X-7-175
Analysis of adenovirus trans-complementation-mediated gene expression controlled by melanoma-specific TETP promoter in vitro
Abstract
Background: Human adenoviruses (Ads) have substantial potential for clinical applications in cancer patients. Conditionally replicating adenoviruses (CRAds) include oncolytic adenoviruses in which expression of the immediate early viral transactivator protein E1A is controlled by a cancer cell-selective promoter. To enhance efficacy, CRAds are further armed to contain therapeutic genes. Due to size constraints of the capsid geometry, the capacity for packaging transgenes into Ads is, however, limited. To overcome this limitation, the employment of E1A-deleted replication-deficient viruses carrying therapeutic genes in combination with replication-competent CRAd vectors expressing E1A in trans has been proposed. Most trans-complementing studies involved transgene expressions from strong ubiquitous promoters, and thereby relied entirely on the cancer cell specificity of the CRAd vector.
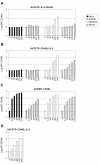
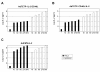
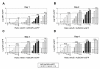
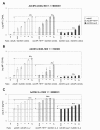
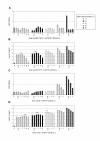
Results: Here we tested the trans-complementation of a CRAd and a replication-deficient transgene vector containing the same cancer cell-selective promoter. Hereto, we generated two new vectors expressing IL-2 and CD40L from a bicistronic expression cassette under the control of the melanoma/melanocyte-specific tyrosinase enhancer tyrosinase promoter (TETP), which we previously described for the melanoma-specific CRAd vector AdDeltaEP-TETP. These vectors gave rise to tightly controlled melanoma-specific transgene expression levels, which were only 5 to 40-fold lower than those from vectors controlled by the nonselective CMV promoter. Reporter analyses using Ad-CMV-eGFP in combination with AdDeltaEP-TETP revealed a high level of trans-complementation in melanoma cells (up to about 30-fold), but not in non-melanoma cells, unlike the AdCMV-eGFP/wtAd5 binary vector system, which was equally efficient in melanoma and non-melanoma cells. Similar findings were obtained when replacing the transgene vector AdCMV-eGFP with AdCMV-IL-2 or AdCMV-CD40L. However, the combination of the novel AdTETP-CD40L/IL-2 vector with AdDeltaEP-TETP or wtAd5 gave reproducible moderate 3-fold enhancements of IL-2 by trans-complementation only.
Conclusions: The cancer cell-selective TETP tested here did not give the expected enforceable transgene expression typically achieved in the Ad trans-complementing system. Reasons for this could include virus-mediated down regulation of limiting transcription factors, and/or competition for such factors by different promoters. Whether this finding is unique to the particular promoter system tested here, or also occurs with other promoters warrants further investigations.
Figures


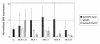
Similar articles
-
Analyses of melanoma-targeted oncolytic adenoviruses with tyrosinase enhancer/promoter-driven E1A, E4, or both in submerged cells and organotypic cultures.Mol Cancer Ther. 2004 Apr;3(4):437-49. Mol Cancer Ther. 2004. PMID: 15078987
-
Novel oncolytic adenoviruses targeted to melanoma: specific viral replication and cytolysis by expression of E1A mutants from the tyrosinase enhancer/promoter.Cancer Res. 2002 Aug 15;62(16):4663-70. Cancer Res. 2002. PMID: 12183423
-
A novel attenuated replication-competent adenovirus for melanoma therapy.Gene Ther. 2003 Apr;10(7):530-9. doi: 10.1038/sj.gt.3301940. Gene Ther. 2003. PMID: 12646858
-
Replicating adenoviruses in cancer therapy.Curr Top Microbiol Immunol. 2004;273:291-334. doi: 10.1007/978-3-662-05599-1_9. Curr Top Microbiol Immunol. 2004. PMID: 14674605 Review.
-
Engineering regulatory elements for conditionally-replicative adeno-viruses.Curr Gene Ther. 2003 Aug;3(4):357-85. doi: 10.2174/1566523034578311. Curr Gene Ther. 2003. PMID: 12871022 Review.
Cited by
-
Homologous recombination-based adenovirus vector system for tumor cell-specific gene delivery.Cancer Biol Ther. 2013 Aug;14(8):728-35. doi: 10.4161/cbt.25090. Epub 2013 Jun 12. Cancer Biol Ther. 2013. PMID: 23792576 Free PMC article.
-
Progress and problems with the use of suicide genes for targeted cancer therapy.Adv Drug Deliv Rev. 2016 Apr 1;99(Pt A):113-128. doi: 10.1016/j.addr.2015.05.009. Epub 2015 May 22. Adv Drug Deliv Rev. 2016. PMID: 26004498 Free PMC article. Review.
-
Multi-color RGB marking enables clonality assessment of liver tumors in a murine xenograft model.Oncotarget. 2017 Dec 14;8(70):115582-115595. doi: 10.18632/oncotarget.23312. eCollection 2017 Dec 29. Oncotarget. 2017. PMID: 29383183 Free PMC article.
-
Gene therapy for advanced melanoma: selective targeting and therapeutic nucleic acids.J Drug Deliv. 2013;2013:897348. doi: 10.1155/2013/897348. Epub 2013 Mar 25. J Drug Deliv. 2013. PMID: 23634303 Free PMC article.
References
-
- Li CY, Huang Q, Kung HF. Cytokine and immuno-gene therapy for solid tumors. Cell Mol Immunol. 2005;2:81–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

