The cyclin A centrosomal localization sequence recruits MCM5 and Orc1 to regulate centrosome reduplication
- PMID: 20663915
- PMCID: PMC2915877
- DOI: 10.1242/jcs.073098
The cyclin A centrosomal localization sequence recruits MCM5 and Orc1 to regulate centrosome reduplication
Abstract
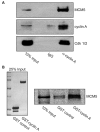
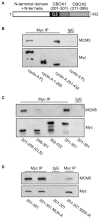
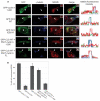
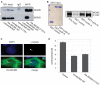
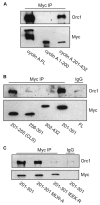
Centrosomes are the major microtubule-organizing centers in animal cells and regulate formation of a bipolar mitotic spindle. Aberrant centrosome number causes chromosome mis-segregation, and has been implicated in genomic instability and tumor development. Previous studies have demonstrated a role for the DNA replication factors MCM5 and Orc1 in preventing centrosome reduplication. Cyclin A-Cdk2 localizes on centrosomes by means of a modular centrosomal localization sequence (CLS) that is distinct from that of cyclin E. Here, we show that cyclin A interacts with both MCM5 and Orc1 in a CLS-dependent but Cdk-independent manner. Although the MRAIL hydrophobic patch is contained within the cyclin A CLS, binding of both MCM5 and Orc1 to cyclin A does not require a wild-type hydrophobic patch. The same domain in MCM5 that mediates interaction with cyclin E also binds cyclin A, resulting in centrosomal localization of MCM5. Finally, unlike its function in DNA synthesis, MCM5-mediated inhibition of centrosome reduplication in S-phase-arrested CHO cells does not require binding to other MCM family members. These results suggest that cyclins E and A sequentially prevent centrosome reduplication throughout interphase by recruitment of DNA replication factors such as MCM5 and Orc1.
Figures

Similar articles
-
Cyclin E-dependent localization of MCM5 regulates centrosome duplication.J Cell Sci. 2008 Oct 1;121(Pt 19):3224-32. doi: 10.1242/jcs.034702. J Cell Sci. 2008. PMID: 18799789
-
Orc1 controls centriole and centrosome copy number in human cells.Science. 2009 Feb 6;323(5915):789-93. doi: 10.1126/science.1166745. Science. 2009. PMID: 19197067 Free PMC article.
-
Meier-Gorlin syndrome mutations disrupt an Orc1 CDK inhibitory domain and cause centrosome reduplication.Genes Dev. 2012 Aug 15;26(16):1797-810. doi: 10.1101/gad.197178.112. Epub 2012 Aug 1. Genes Dev. 2012. PMID: 22855792 Free PMC article.
-
Same partners, different dance: involvement of DNA replication proteins in centrosome regulation.Cell Cycle. 2010 Nov 15;9(22):4487-91. doi: 10.4161/cc.9.22.14047. Epub 2010 Nov 15. Cell Cycle. 2010. PMID: 21088489 Review.
-
P53, cyclin-dependent kinase and abnormal amplification of centrosomes.Biochim Biophys Acta. 2008 Sep;1786(1):15-23. doi: 10.1016/j.bbcan.2008.04.002. Epub 2008 Apr 22. Biochim Biophys Acta. 2008. PMID: 18472015 Free PMC article. Review.
Cited by
-
Genome wide comparative comprehensive analysis of Plasmodium falciparum MCM family with human host.Commun Integr Biol. 2012 Nov 1;5(6):607-15. doi: 10.4161/cib.21759. Commun Integr Biol. 2012. PMID: 23336032 Free PMC article.
-
Predicting embryonic aneuploidy rate in IVF patients using whole-exome sequencing.Hum Genet. 2022 Oct;141(10):1615-1627. doi: 10.1007/s00439-022-02450-z. Epub 2022 Mar 26. Hum Genet. 2022. PMID: 35347416 Free PMC article.
-
Mining the Giardia genome and proteome for conserved and unique basal body proteins.Int J Parasitol. 2011 Aug 15;41(10):1079-92. doi: 10.1016/j.ijpara.2011.06.001. Epub 2011 Jul 1. Int J Parasitol. 2011. PMID: 21723868 Free PMC article.
-
The centrosome and its duplication cycle.Cold Spring Harb Perspect Biol. 2015 Feb 2;7(2):a015800. doi: 10.1101/cshperspect.a015800. Cold Spring Harb Perspect Biol. 2015. PMID: 25646378 Free PMC article. Review.
-
Par6γ is at the mother centriole and controls centrosomal protein composition through a Par6α-dependent pathway.J Cell Sci. 2013 Feb 1;126(Pt 3):860-70. doi: 10.1242/jcs.121186. Epub 2012 Dec 21. J Cell Sci. 2013. PMID: 23264737 Free PMC article.
References
-
- Berthet C., Aleem E., Coppola V., Tessarollo L., Kaldis P. (2003). Cdk2 knockout mice are viable. Curr. Biol. 13, 1775-1785 - PubMed
-
- Bettencourt-Dias M., Glover D. M. (2007). Centrosome biogenesis and function: centrosomics brings new understanding. Nat. Rev. Mol. Cell Biol. 8, 451-463 - PubMed
-
- Chen Z., Indjeian V. B., McManus M., Wang L., Dynlacht B. D. (2002). CP110, a cell cycle-dependent CDK substrate, regulates centrosome duplication in human cells. Dev. Cell 3, 339-350 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

